Clinical trial overview
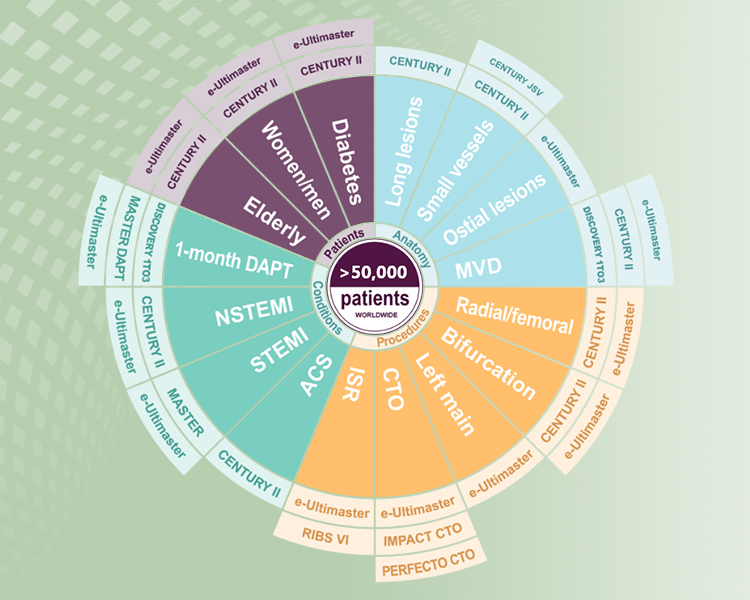
> 50,000 patients completed in the Ultimaster™ clinical programs.1-3
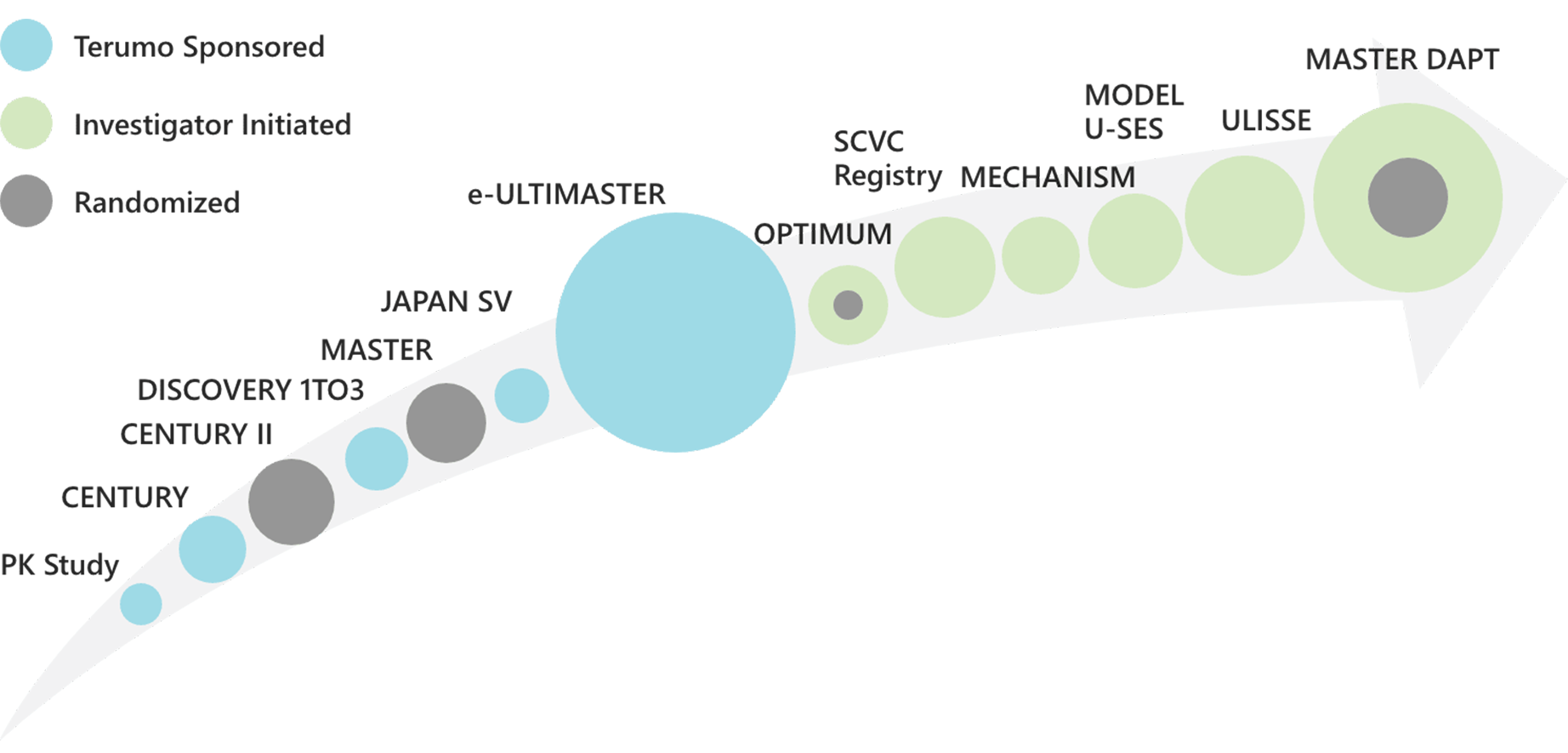
| Study name | Study type | Patients | Endpoint | Status | Reference |
|---|---|---|---|---|---|
| TCD-10023PK | Single arm, Pharmaco-kinetics | 20 | Sirolimus concentration at 28 days | Completed | Pubmed |
| CENTURY | Single arm, First in human | 104 | Late lumen loss at 6 month | Completed 5 year FU | Pubmed Presentation |
| CENTURY II | Randomized clinical trial vs Xience | 1123 | Freedom from Target lesion failure at 9month | Completed 5 year FU | Pubmed Presentation |
| Bifurcation lesion | Subgroup analysis | 194 | - | - | Pubmed Presentation (Slide.18-19) |
| Multivessel disease | Subgroup analysis | 456 | - | - | Pubmed |
| High risk ACS | Subgroup analysis | 260 | - | - | Pubmed |
| Long lesion | Subgroup analysis | 110 | - | - | Pubmed |
| Small vessel | Subgroup analysis | 514 | - | - | Pubmed |
| NSTEMI | Subgroup analysis | 203 | - | - | Presentation |
| Vascular response by OCT evaluation | Subgroup analysis | 27 | - | - | Pubmed |
| MASTER | Randomized clinical trial vs BMS(Kaname) | 500 | Safety at 1month, efficacy at 6month, efficacy and safety at 12 month | Completed 3 year FU | Pubmed/ Presentation |
| DISCOVERY 1TO3 | Single arm | 60 | Stent strut coveredge at 3month | Completed 1 year FU | Pubmed |
| CENTURY JSV | Single arm | 70 | MACE at 9 month | Completed 2 year FU | Pubmed |
| e-Ultimaster | All-comers registry | 37,198 | Target lesion failure at 1 year | Complete 1 year FU | Presentation |
| LMT | Subgroup analysis | 1,099 | - | - | - |
| Bifurcation | Subgroup analysis | 4,395 | - | - | Pubmed |
| Multivessel disease | Subgroup analysis | 16,267 | - | - | Presentation |
| Complex PCI | Subgroup analysis | 9,793 | - | - | Pubmed |
| Proximal LAD | Subgroup analysis | 5,452 | - | - | Pubmed |
| CKD | Subgroup analysis | 1,466 | - | - | Pubmed |
| MECHANISM UM | Multicenter single arm registry | 200 | Vascular healing response at 1month, 3month and 12 month | Completed 1 year FU | Pubmed |
| MODEL U-SES | Multicenter single arm registry | 1500 | Ischemic and bleeding event at 12 month | Completed 1 year FU | Pubmed |
| HBR | Subgroup analysis | 1,695 | - | - | Pubmed |
| OPTIMUM | Multicenter single arm registry | 106 | Incomplete stent apposition rate on OFDI after stent implantation | Completed | Pubmed |
| Sapporo cardio vascular clinic report | All comer, single center registry | 1727 | TLR and TLF | Completed 1 year FU | Pubmed |
| ULISSE registry | Multicenter single arm registry | 1,660 | Target lesion failure at 1 year | Completed 1 year FU | Pubmed |
| AMI | Subgroup analysis | 381 | - | - | Pubmed |
| Diabetes | Subgroup analysis | 485 | - | - | Pubmed |
| Short DAPT | Subgroup analysis | 82 | - | - | Pubmed |
| MASTER DAPT | Randomized clinical trial 1month DAPT vs regular DAPT | 4,300 | NACE, MACCE and MCB at 11 month | Completed | Pubmed |
MASTER DAPT
The largest, multi-center randomized controlled study on the use of abbreviated vs short DAPT with Ultimaster™ stent. The results released at ESC 2021 showed short DAPT to be noninferior to standard therapy with regard to NACE and MACCE. It also resulted in a lower incidence of major or clinically relevant non-major bleeding.4
Disclaimer
MASTER DAPT study is sponsored by the European Cardiovascular Research Institute (ECRI, Rotterdam, The Netherlands) and supported with a restricted research grant by Terumo Europe. The study is managed by global CROs and data management group (CERC, Paris, France, Cardialysis, Rotterdam, The Netherlands, CV quest. Co. Ltd., Tokyo, Japan and CTU, Bern, Switzerland).
e-Ultimaster
e-Ultimaster is one of the largest real-world registries with more than 37,000 patients. Results demonstrated Ultimaster to be safe and effective even in complex cases. Multiple sub-analysis showed excellent clinical outcomes for patients with bifurcation lesions, multivessel disease and more.5
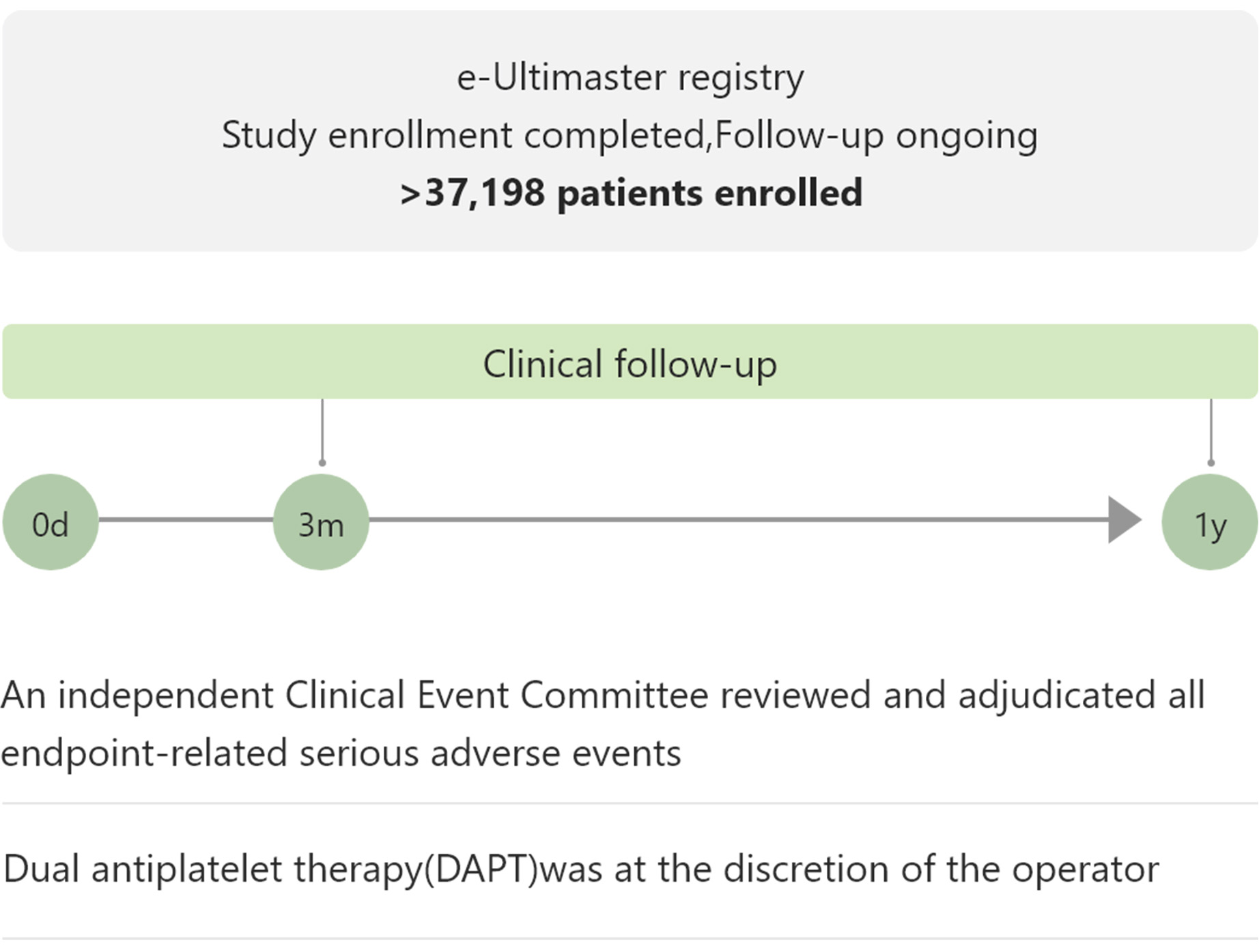
An independent Clinical Event Committee reviewed and adjudicated all endpoint-related serious adverse events
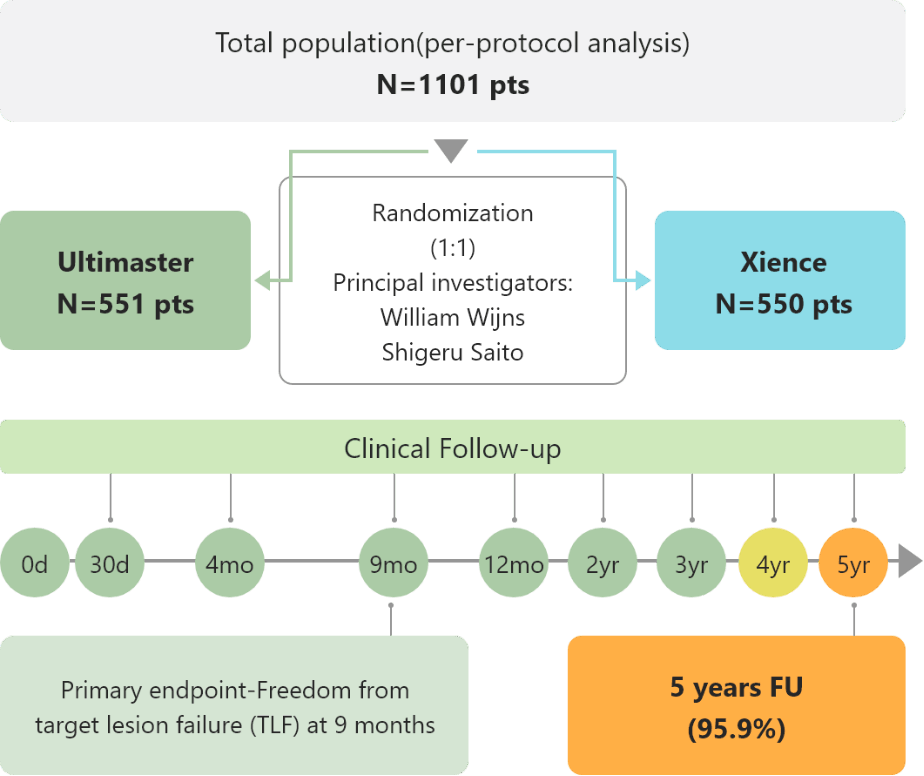
CENTURY II
CENTURY II is a large-scale, prospective, multicenter, randomized single-blind, controlled, non-inferiority trial. It proves Ultimaster™ stent to be noninferior to Xience, with safety and efficacy comparable up to five-year follow-up after PCI.6
All clinical evidences of Ultimaster™