CENTURY II
Overview
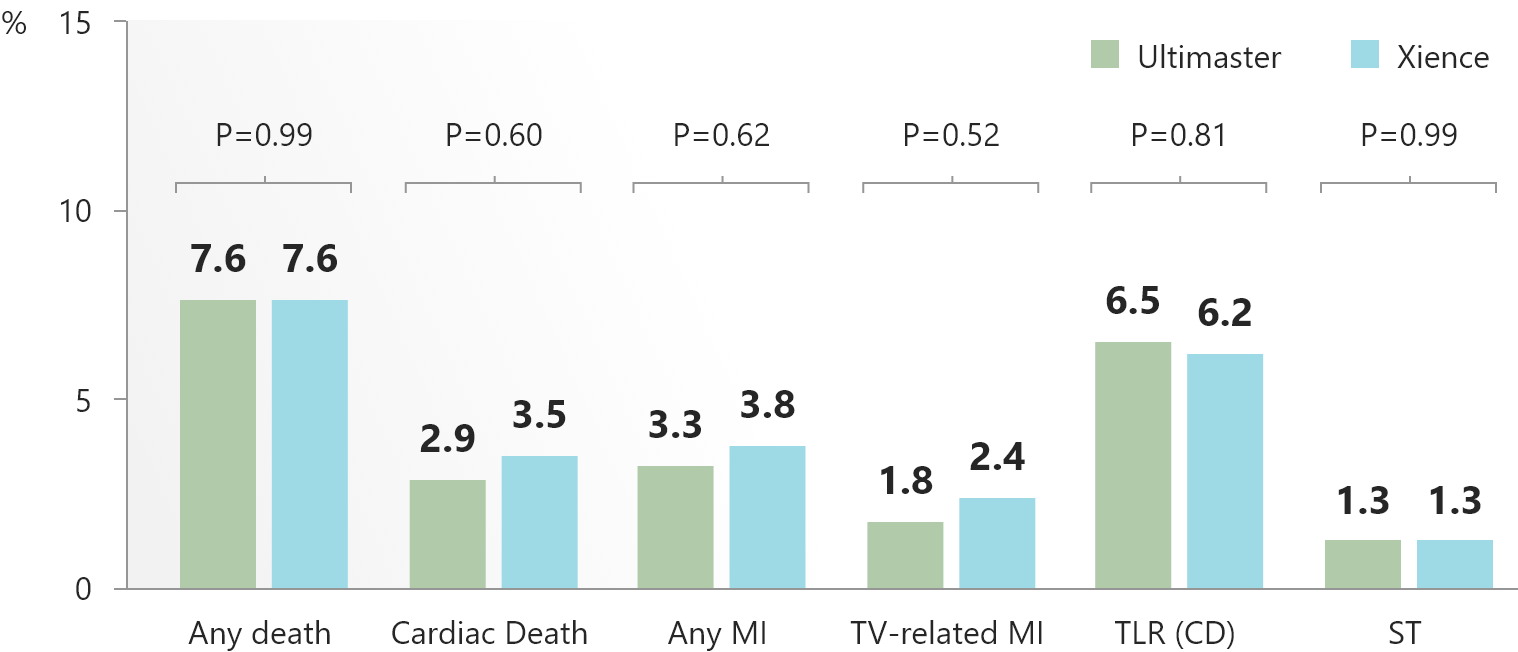
- Comparable clinical outcomes of Ultimaster™ stent with bioresorable polymer coating versus Xience stent with durable polymer coating are maintained up to five years.
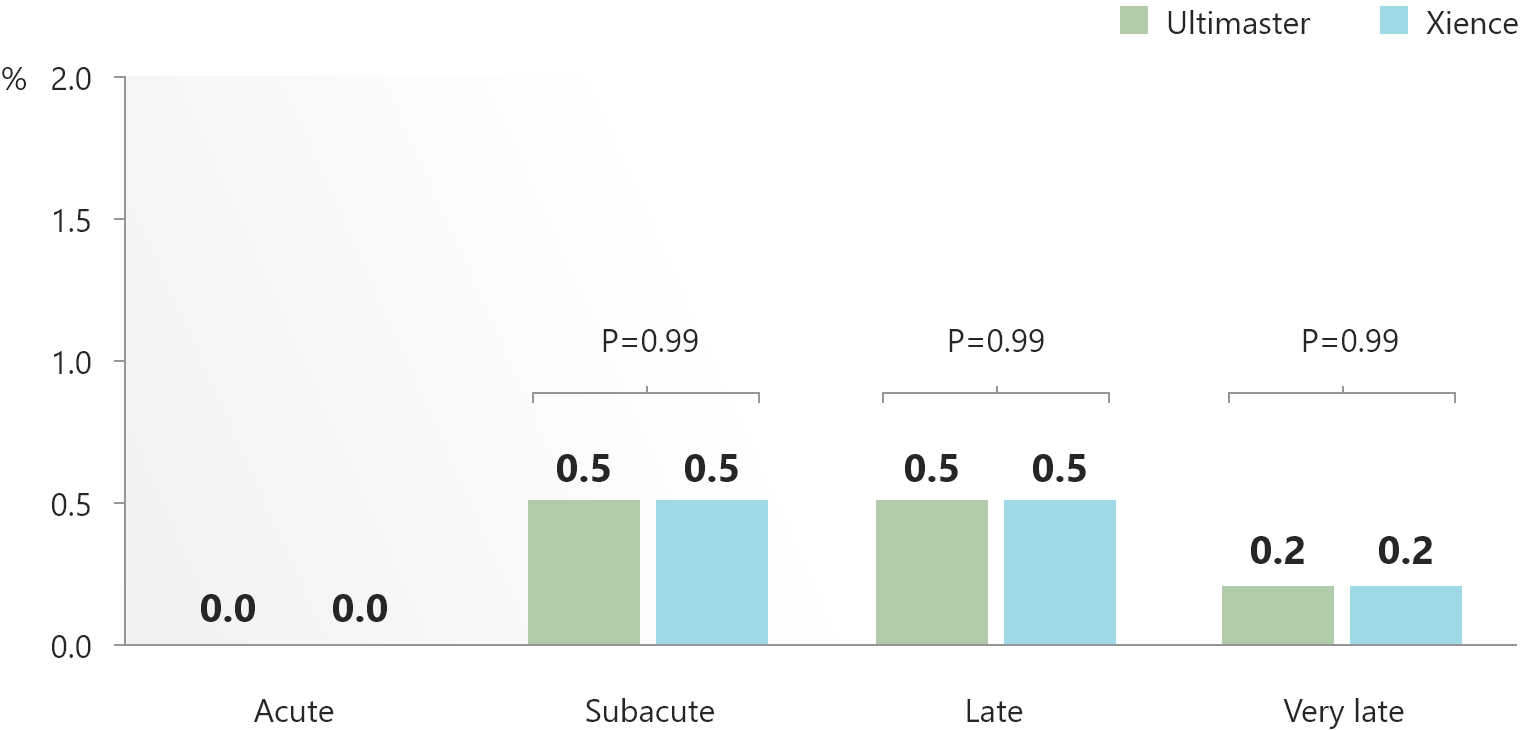
- Particularly remarkable was the low rate of very late stent thrombosis (0.2%) in both arms.
- These data supports the long term safe use and good performance of the Ultimaster™ DES.
Study Design
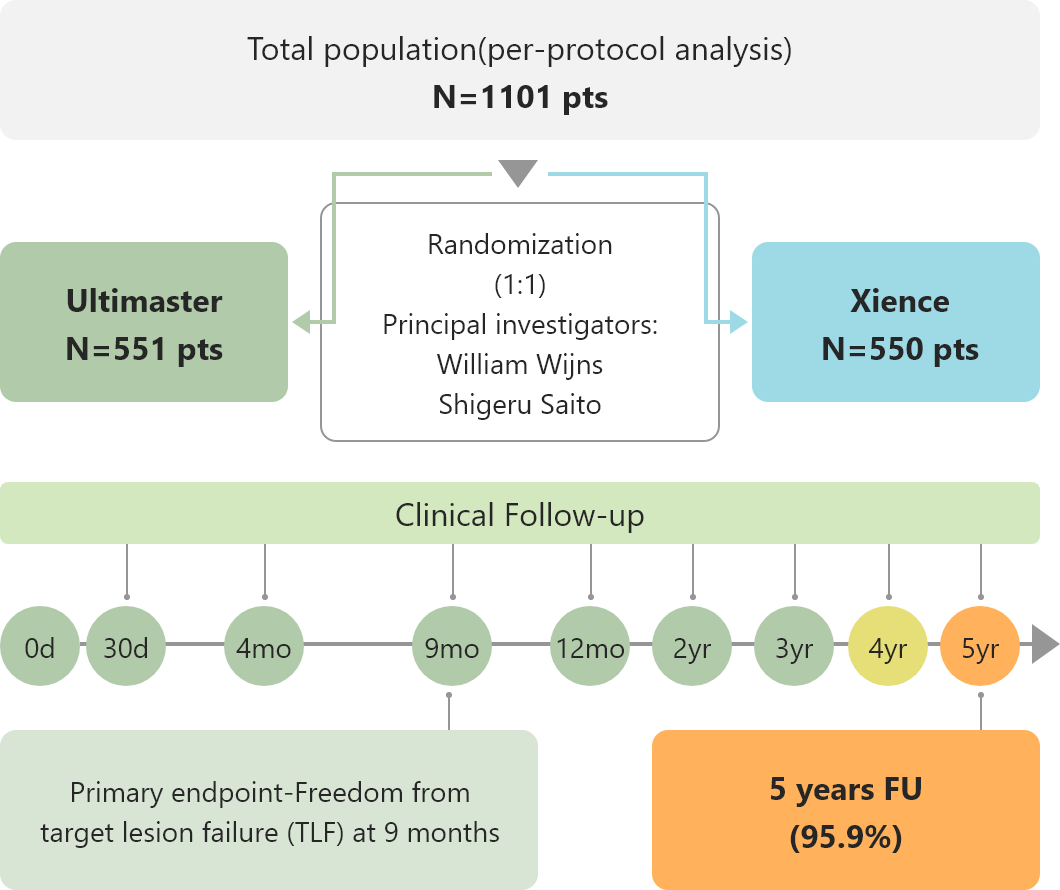
Study Outline
Patient Background
| Ultimaster N=551 pts | Xience N=550 pts | P | |
|---|---|---|---|
| Age, years (mean ±SD) | 65.2+10.5 | 65.5+10.6 | 0.61 |
| Gender - male, % | 78.6 | 82.4 | 0.11 |
| DM, % | 31.9 | 30.9 | 0.71 |
| IDDM, % | 16.5 | 14.7 | 0.65 |
| Hypertension, % | 73.3 | 67.8 | 0.05 |
| Current Smoker, % | 22.2 | 23.9 | 0.50 |
| Previous smaker, % | 46.7 | 42.0 | 0.12 |
| Previous MI, % | 28.3 | 27.6 | 0.80 |
| Previous PCI, % | 37.2 | 35.0 | 0.45 |
| Previous CABG, % | 4.5 | 3.7 | 0.46 |
| Peripheral vascular discase, % | 9.6 | 6.6 | 0.06 |
| High risk ACS, % | 22.5 | 24.7 | 0.39 |
| Ultimaster N=551 pts Nlesions=711 | Xience N-550 pts Nlesions=716 | P | |
|---|---|---|---|
| Multi-vessel disease, % | 39.6 | 41.3 | 0.56 |
| Lesions detected ,n | 2.0±1.3 | 2.0±1.3 | 0.67 |
| Lesions treated,n | 1.3±0.6 | 1.3±0.6 | 0.62 |
| Bifurcation/lesion, % | 13.8 | 14.4 | 0.74 |
| Ostial/lesion, % | 6.0 | 8.4 | 0.08 |
| Moderate/severe calcification, % | 21.5 | 17.7 | 0.70 |
| Access site, % Radial Femoral | 71.7 26.7 | 73.1 25.6 | 0.55 |
| N° of stents implanted/pt | 1.5±0.8 | 1.6±0.9 | 0.94 |
| Total implanted stent length/pt | 29.5±17.0 | 29.6±18.1 | 0.66 |
| Delivery success, % | 99.1 | 99.5 | 0.23 |
| Procedure success/pt, % | 98.0 | 98.2 | 0.83 |
Result
5-year clinical outcomes