MASTER DAPT OAC


Overview
Investigator-initiated
global randomized study
global randomized study
4,579 patients,
140 hospital worldwide, 30 countries
140 hospital worldwide, 30 countries
All-comers trial in
HBR patients after PCI
HBR patients after PCI
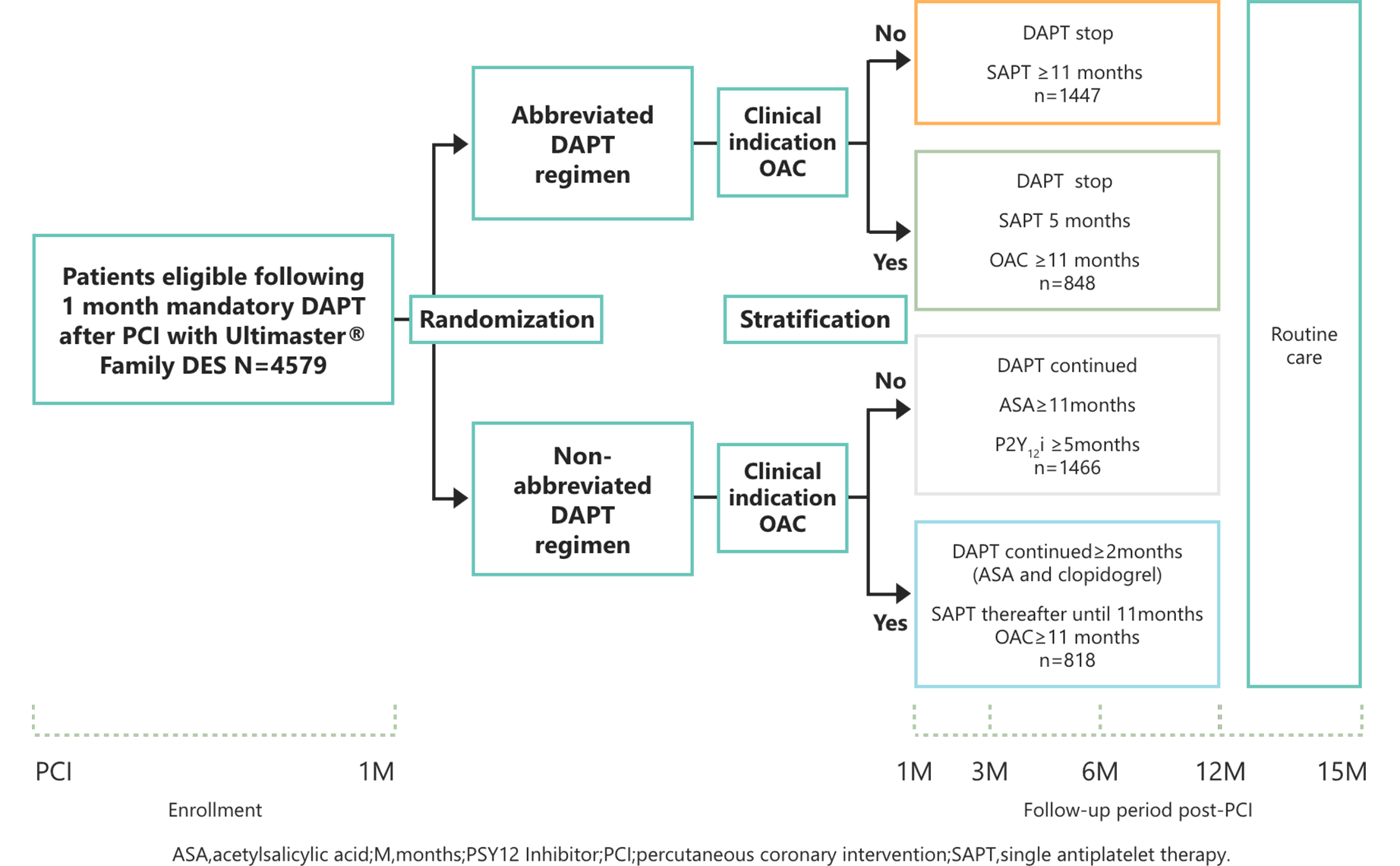
Abbreviated vs prolonged
DAPT in patients treated with
the Ultimaster™and Ultimaster™ Tansei™ DES
DAPT in patients
treated with the
Ultimaster™ and
Ultimaster™Tansei™
DES
treated with the
Ultimaster™ and
Ultimaster™Tansei™
DES
Study Design
Primary Endpoint
- Net adverse clinical events (NACE) defined as a composite of death from any cause, myocardial infarction, stroke or major bleeding.
- Major adverse cardiac and cerebrovascular events (MACCE), defined as a composite of death from any cause, myocardial infarction, stroke or major bleeding.
- Major or clinically relevant non-major bleeding (MCB), defined as BARC 2, 3 or 5 bleeding.
Result
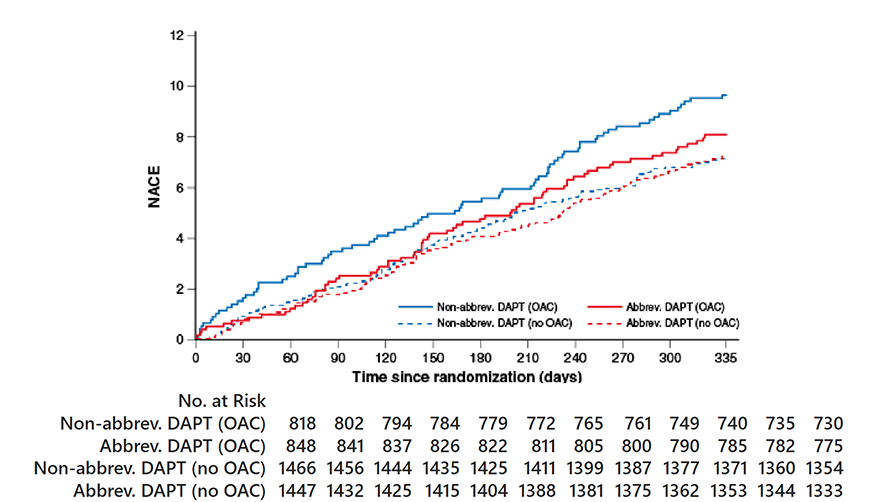
NACE
OAC indication HR 0.83, 95% CI 0.60-1.15;
p=0.26 No OAC indication HR HR 1.01, 95% CI 0.77-1.33;
p=0.91 Pinteraction=0.28
p=0.26 No OAC indication HR HR 1.01, 95% CI 0.77-1.33;
p=0.91 Pinteraction=0.28
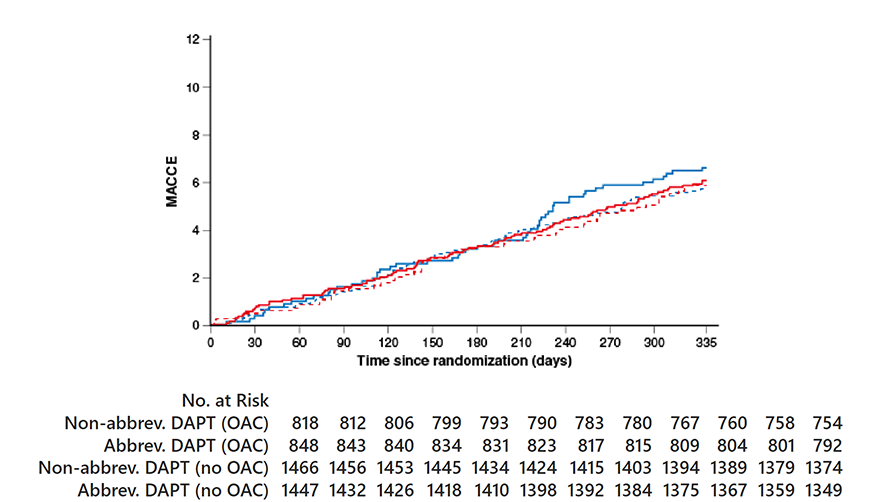
MACCE
OAC indication HR0.88, 95% CI 0.60-1.30;
p=0.53 No OAC indication HR 1.06, 95% CI 0.79-1.44;
p=0.67 Pinteraction=0.46
p=0.53 No OAC indication HR 1.06, 95% CI 0.79-1.44;
p=0.67 Pinteraction=0.46
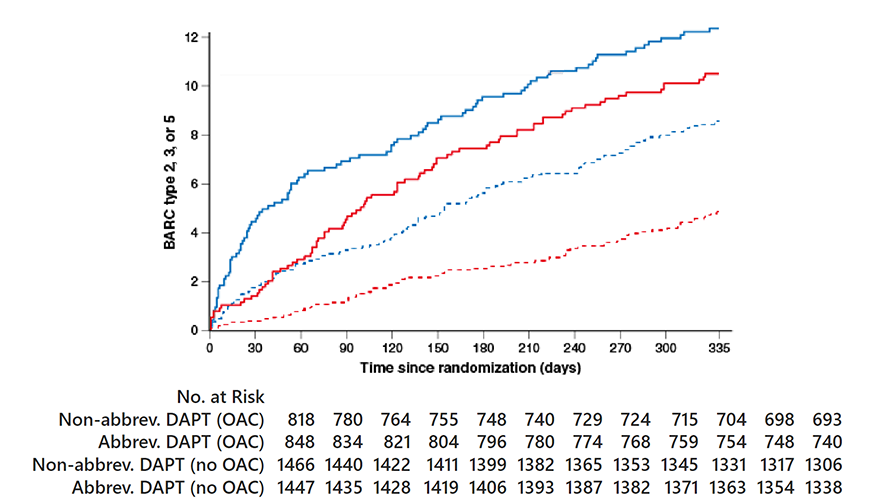
Major or clinically relevant non-major bleeding
OAC indication HR 0.83, 95% CI 0.62-1.12; p=0.25
No OAC indication HR 0.55, 95% CI 0.41-0.74; p<0.001P
Pinteraction=0.06
No OAC indication HR 0.55, 95% CI 0.41-0.74; p<0.001P
Pinteraction=0.06
MASTER DAPT study is sponsored by the European Cardiovascular Research Institute (ECRI, Rotterdam, The Netherlands) and supported with a restricted research grant by Terumo Europe.