Special Topics
MASTER DAPT Summary
Click here to download the summary of MASTER DAPT.
Learning from Experts
Get insights from Interview about optimal DAPT duration with Dr. Muramatsu.
MASTER DAPT Study Highlights
Investigator-initiated
global randomized study
global randomized study
4,579 patients,
140 hospital worldwide, 30 countries
140 hospital worldwide, 30 countries
All-comers trial in
HBR patients after PCI
HBR patients after PCI
Abbreviated vs prolonged
DAPT in patients treated with
the Ultimaster™and Ultimaster™ Tansei™
DAPT in patients
treated with the
Ultimaster™ and
Ultimaster™Tansei™
treated with the
Ultimaster™ and
Ultimaster™Tansei™
To receive more information about the clinical study or our product,
We will contact you after your request.
Study Design
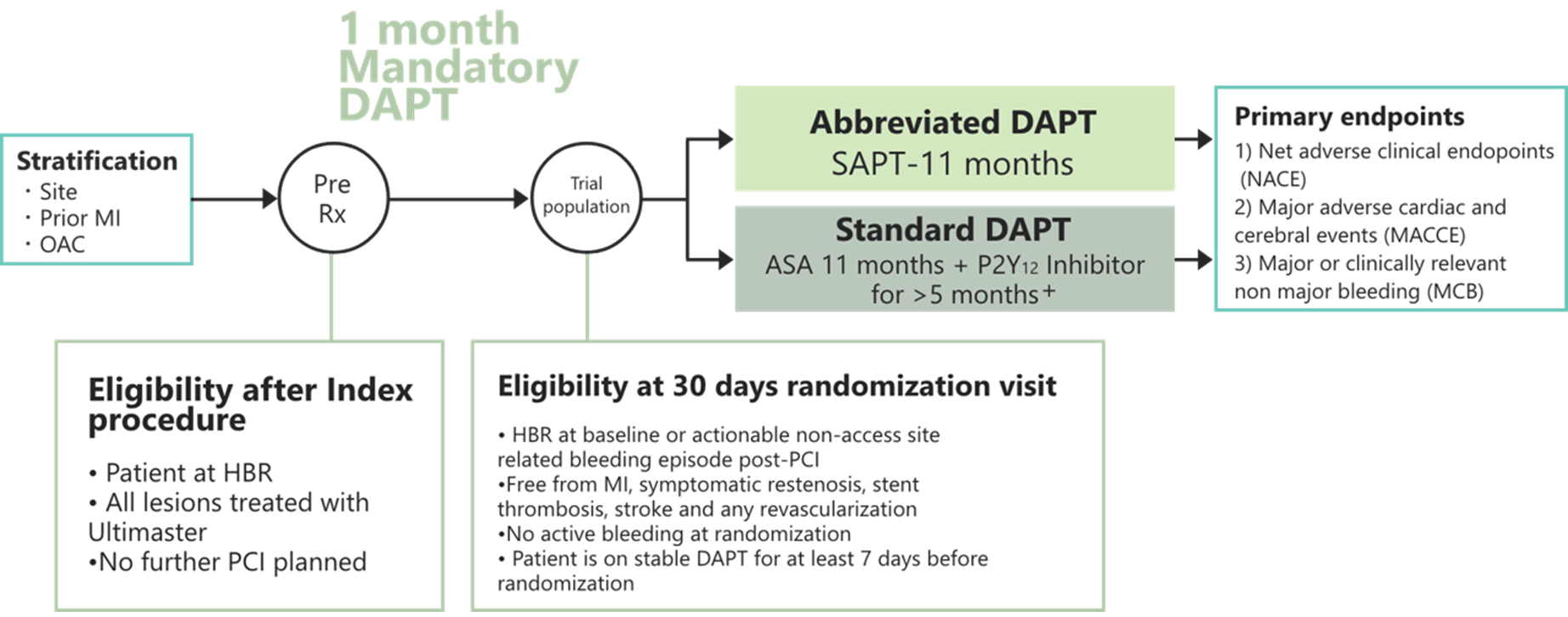
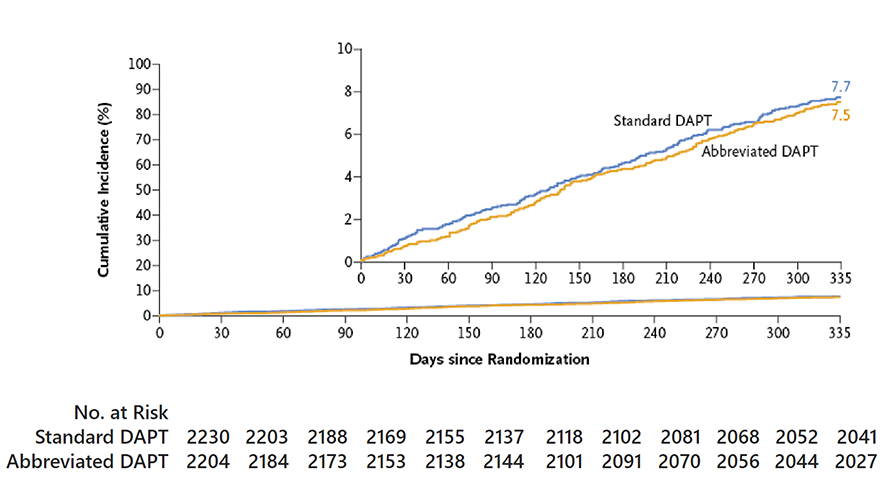
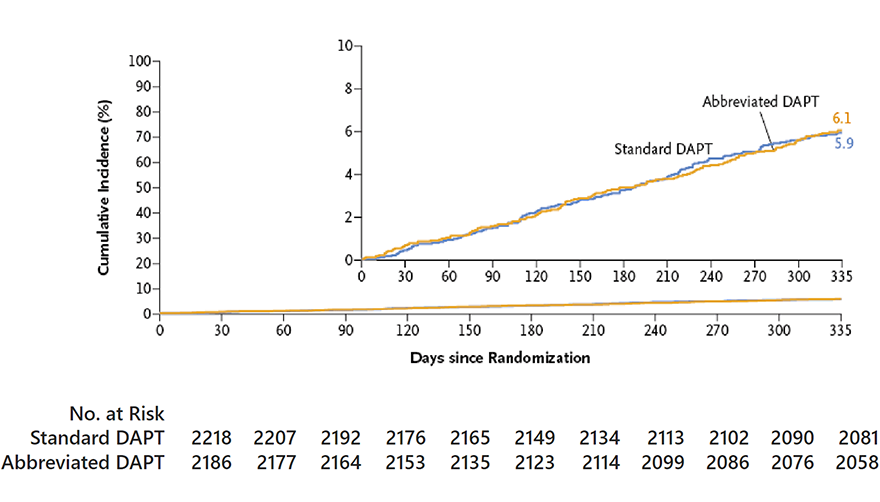
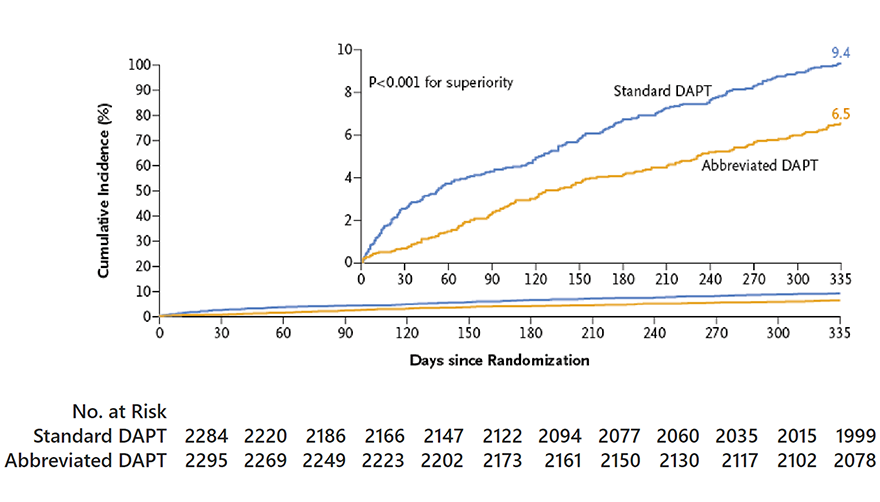
MASTER DAPT Primary Endpoints
MASTER DAPT study had three primary endpoints
Net adverse clinical events (NACE) defined as a composite of death from any cause, myocardial infarction, stroke or major bleeding.
Major or clinically relevant non-major bleeding (MCB), defined as BARC 2, 3 or 5 bleeding.
Major adverse cardiac and cerebrovascular events (MACCE), defined as a composite of death from any cause, myocardial infarction, stroke or major bleeding.
To receive more information about the clinical study or our product,
We will contact you after your request.
To receive more information about the clinical study or our product,
We will contact you after your request.
MASTER DAPT study is sponsored by the European Cardiovascular Research Institute (ECRI, Rotterdam, The Netherlands) and supported with a restricted research grant by Terumo Europe.
Special Topics
The Summary of MASTER DAPT
Dr.Marco at ESC Congress 2021
MASTER DAPT Sub-group Analysis
Clinical Evidence of Ultimaster™
Approved for 16 CE marks, the Ultimaster™ DES has the most extensive clinical trial data with more than 50,000 patients completed worldwide.2-4
To receive more information about the clinical study or our product,
We will contact you after your request.