e-Ultimaster Left Main sub-analysis
Overview
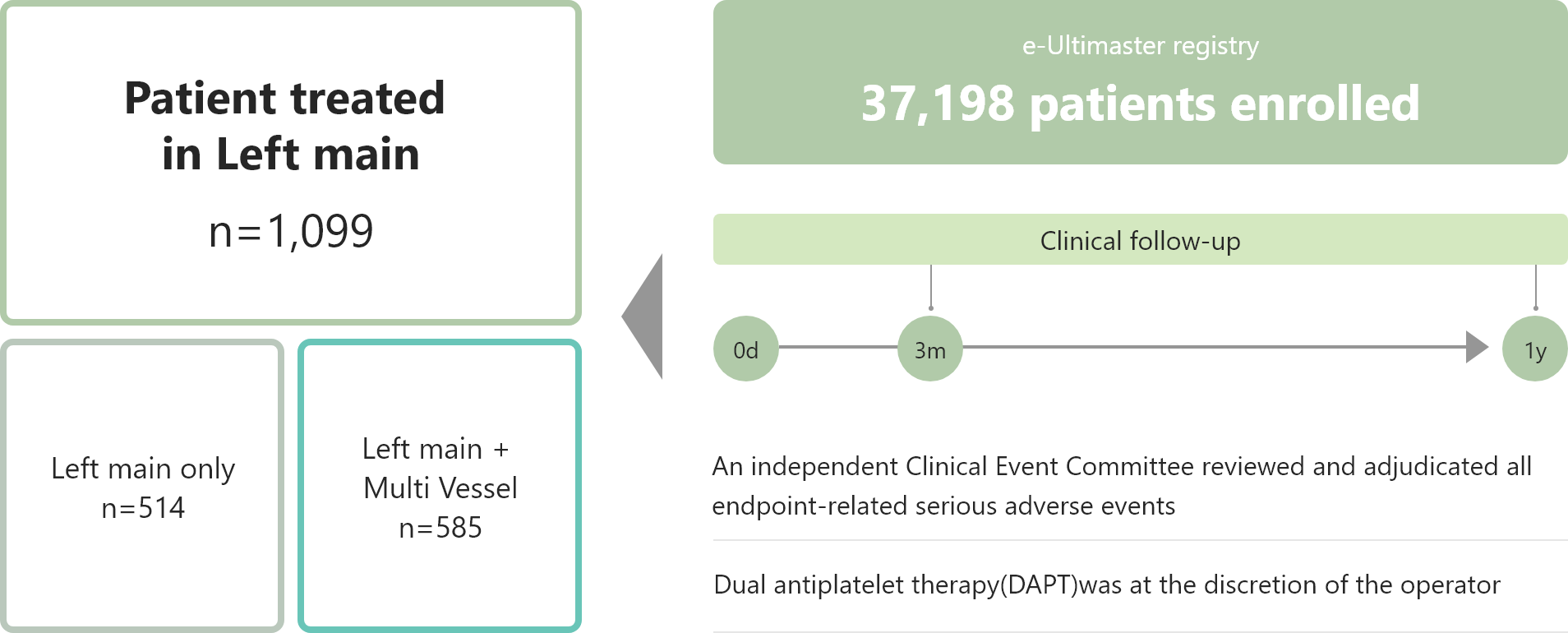
・1,099 patients with Left main lesions treated with Ultimaster™ DES showed acceptable clinical outcomes.
Study Design
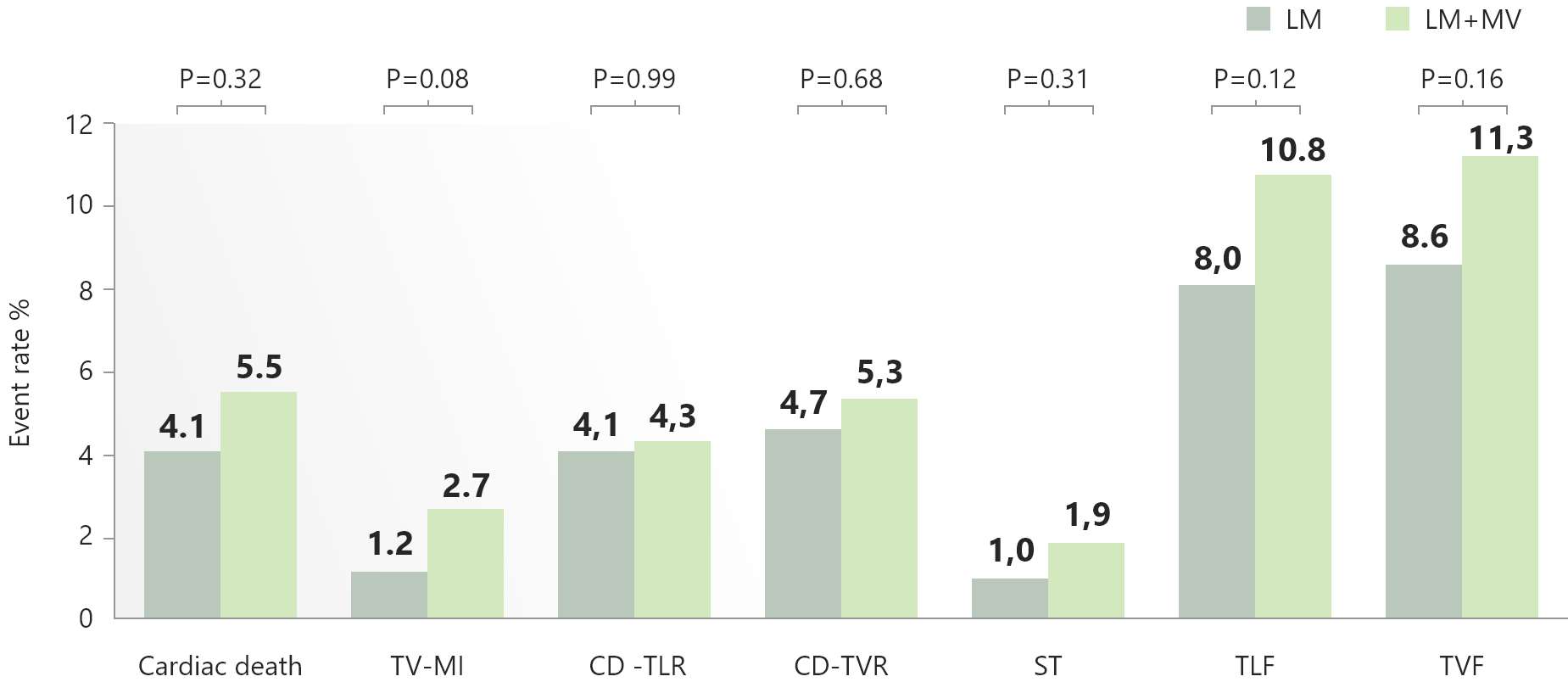
Primary Endpoint
Target lesion failure at 1 year
・Cardiac death, target vessel MI or clinically driven target lesion revascularization
Study Outline
Patient background
| Patient characteristics | LM only n= 514 | LM + MV n= 585 | p-value |
|---|---|---|---|
| Mean age, year | 69.2±11.0 | 68.9±10.9 | P=0.65 |
| BMI kg/m² | 27.2±5.1 | 27.6±5.6 | P=0.25 |
| Hyperlipidaemia % | 59.6 | 63.3 | P=0.23 |
| Diabetes, % | 33.7 | 33.2 | P=0.90 |
| Hypertension, % | 74.7 | 71.6 | P=0.27 |
| Previous PCI % | 39.5 | 36.9 | P=0.41 |
| Previous CABG% | 22 | 18 | P=0.10 |
| Patient characteristics | LM only n= 514 | LM + MV n= 585 | p-value |
|---|---|---|---|
| Lesion longer > 25 mm % | 44.8 | 56.1 | P<0.001 |
| Calcified | 32.2 | 32.56 | P<0.001 |
| Num of stents/pt, n | 1.4± 0.7 | 2.2 ±1.2 | P<0.0001 |
| Total stent length/pt, mm | 30.0± 20.3 | 48.9± 32.2 | P<0.0001 |
| Syntax < 22 % | 68.8 | 42.4 | P<0.0001 |
| Syntax 23-32 | 21.9 | 40.1 | P=0.001 |
| Post dilatation % | 69.7 | 74.9 | P=0.06 |
| Radial access % | 68.5 | 68 | P=0.90 |
| OFDI + IVUS % | 36.5 | 27.1 | P<0.01 |
| Direct stenting % | 29.4 | 51.3 | P<0.0001 |
Result