MODEL U-SES
Overview
・MODEL U-SES trial demonstrated that 3-month DAPT was non-inferiority to adjusted cohort of longer DAPT after BP-SES implantation in net adverse clinical events.
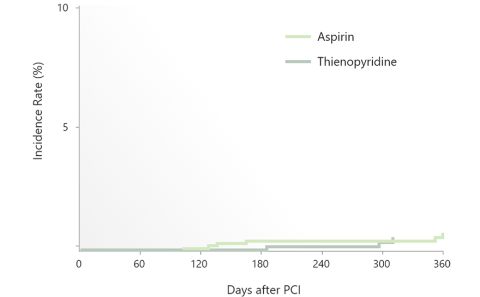
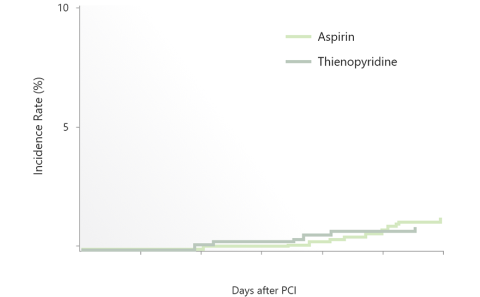
・P2Y12 inhibitor monotherapy was almost equivalent to Aspirin monotherapy after 3 months in terms of both bleeding and thrombotic events.
Study Design
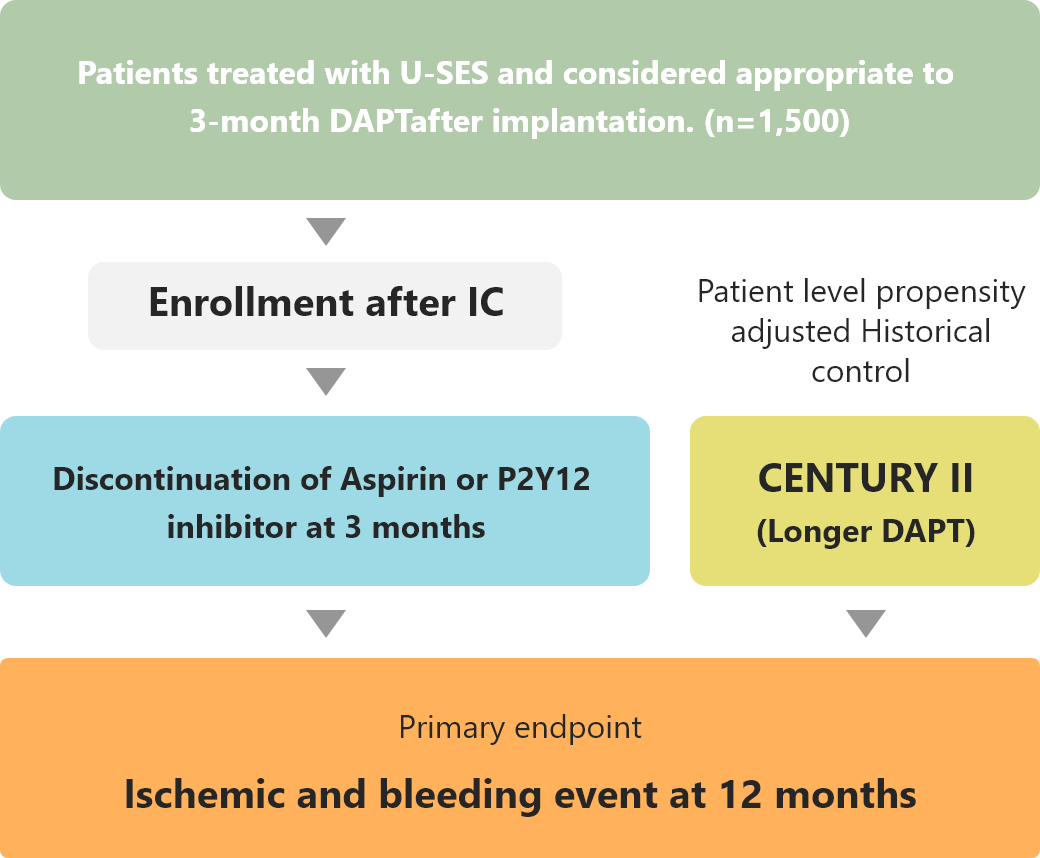
Study Outline
Patient background comparison with historical control
| MODEL U-SES (n=1,695) | CENTURY II (n=549) | p-Value | |
|---|---|---|---|
| Age, years | 69.7 10.6 | 65.3 10.5 | < 0.001 |
| Age >75 years | 36.2% | 19.7% | < 0.001 |
| Male gender | 76.8% | 78.5% | 0.41 |
| Body mass index, kg/m? | 24.313.5 | 27.0 4.1 | < 0.001 |
| Diabetes | 39.3% | 32.2% | 0.003 |
| Hypertension | 79.9% | 73.7% | 0.002 |
| Current smoker | 21.7% | 22.4% | 0.72 |
| Severe CKD | 13.0% | 4.0% | < 0.001 |
| Hemodialysis | 5.3% | 2.0% | 0.001 |
| Prior stroke | 10.4% | 3.8% | < 0.001 |
| Heart failure | 27.2% | 29.3% | 0.35 |
| Peripheral vascular disease | 6.7% | 9.7% | 0.03 |
Result
Landmark analysis: Efficacy and Safety
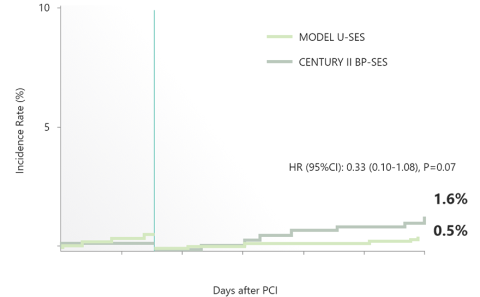
Landmark Analysis at 90 days:
Cardiovascular death, MI, Stroke and ST
With Propensity Score Adjustment
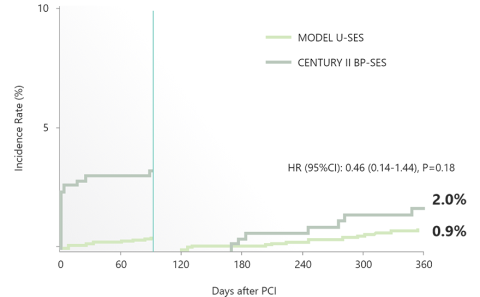
Landmark Analysis at 90 days:
Bleeding (BARC 3 or 5)
With Propensity Score Adjustment
Comparison of Single Antiplatelet Therapy: Aspirin and P2Y12 receptor inhibitor
Landmark Analysis at 90 days:
Cardiovascular death, MI, Stroke and ST
With IPTW Analysis
Landmark Analysis at 90 days:
Bleeding (BARC 3 or 5)
With IPTW Analysis