1980’s
1985
Product Launch
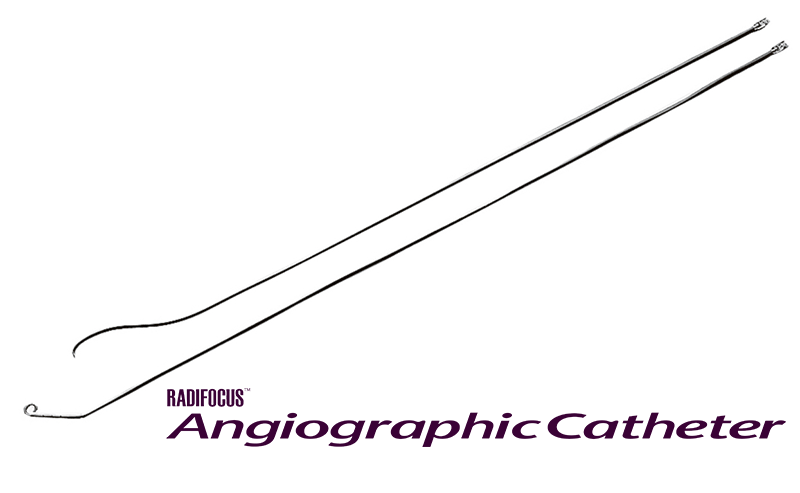
RADIFOCUS™ Guide Wire M RADIFOCUS™ Angiographic Catheter
Starts making products for interventional medicine and launches the RADIFOCUS™ Guide Wire, which continues to be an important tool in this field.

1986
Product Launch
RADIFOCUS™ Introducer kit
Adds RADIFOCUS™ Introducer Sheath to product portfolio.
1989
Product Launch
RADIFOCUS™ SP Catheter
Launches the first made-in-Japan micro-catheter.
1990’s
1990
Product Launch
AnplastC PTCA Dilatation Catheter
Launches the first made-in-Japan PTCA dilatation catheter. At this stage, the product is only available at a limited number of facilities.
1991
Global Network
Start of US Sales
Concludes a distribution agreement with Boston Scientific and starts the sale of access products in the US.
1995
Global Network
Development of TRI Products
Starts developing products to support interventional procedures via radial access.
Product Launch
Stenofocus™ NT PTCA Dilatation Catheter
Begins Japan-wide sales of PTCA dilatation catheter.

Organization
Catheter Terumo Business Unit
Officially establishes a business unit dedicated to the sale of catheters.
1999
Product Launch
Terumo Stent
Develops the first made-in-Japan coronary stent and names it Terumo Stent.

Product Launch
Hayate™
Begins overseas sales of PTCA dilatation catheters, starting with the European market.

2000’s
2000
Product Launch
Intra Imaging Sytem
Complements catheter and wire products by launching its first intravascular ultrasound (IVUS) system.
Product Launch
Progreat™
Launches Progreat™ microcatheter for abdominal diagnosis and treatment. It continues to be used by physicians around the world.

2002
Training
Terumo Medical Pranex
Launches training facilities with the aim of developing and disseminating medical techniques. This marks the start of Terumo’s emphasis on not only products but also training.

Organization
Terumo Group Company System
Launches a group company specializing in catheters, marking a change in organizational structure that will further enhance the interventional systems business.
2003
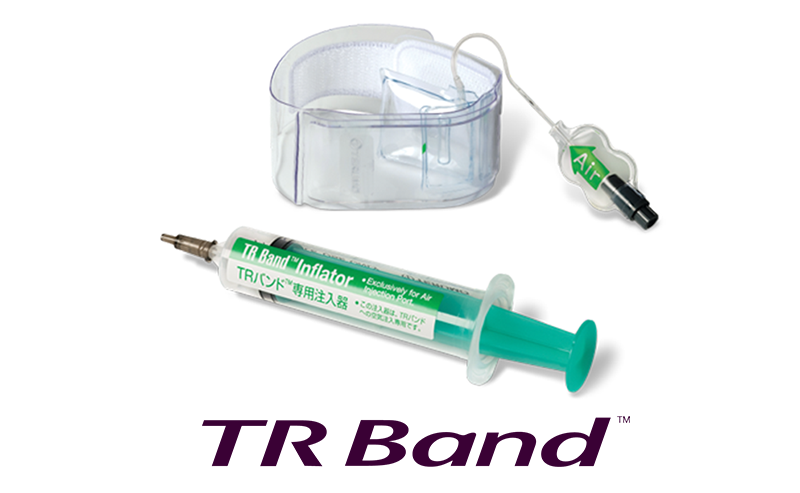
Product Launch
TR Band™
Launches the TR Band™, thereby establishing a full product portfolio that covers all interventional procedures, from access to closure.
Product Launch
Runthrough™ NS PTCA Guidewire
Launches the Runthrough™ NS, a PTCA guidewire with a directly jointed stainless steel and nitinol shaft that continues to be trusted by physicians around the world.

2006
Global Network
Direct Sales in US market
Begins direct sales of products in the US, rather than going through a local distributor. This marks an important milestone in the globalization of the interventional systems business.

2007
Product Launch
Misago™
Develops its first self-expandable peripheral stent and launches it in the European market.

Product Launch
AZUR™
Launches a peripheral hydrogel coil featuring a unique hydrogel technology.

2008
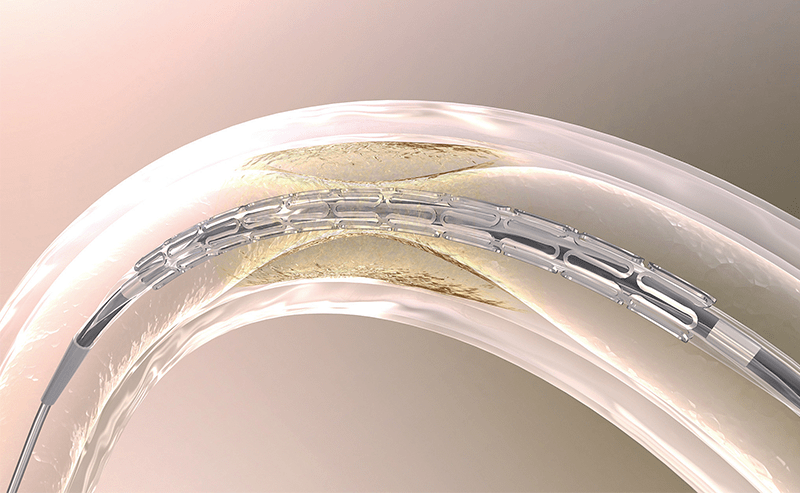
Product Launch
Nobori™
Becomes the first Japanese company to produce a drug eluting stent (DES) and launches it in the European market. Sales of the Nobori™ DES is subsequently expanded worldwide.
Global Network
Clinical Supply Co., Ltd. Added
Adds Clinical Supply to the Group, leading to the expansion of interventional oncology product portfolio.
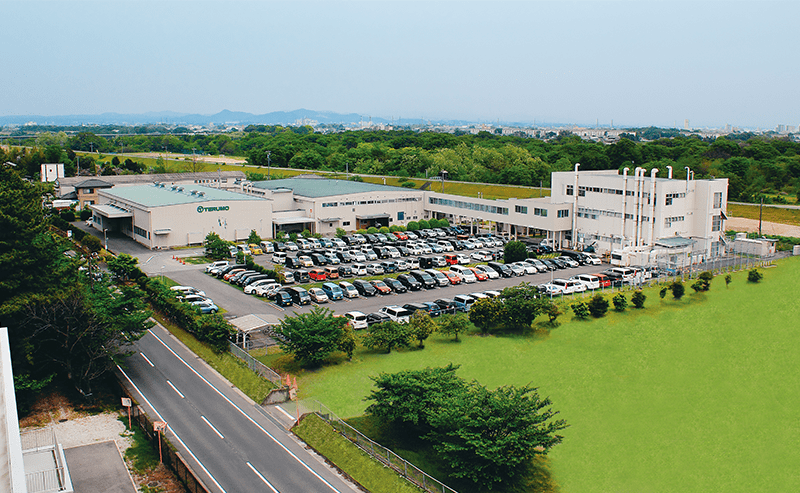
Global Network
Terumo Vietnam Co., Ltd.
Continues to globalize and opens a factory in Vietnam to serve as a production base for supplying products to Asia and Latin America.

2010’s
2010
Organization
Porter Prize
Wins the Porter Prize, awarded by Hitotsubashi University Business School’s School of International Corporate Strategy (Hitotsubashi ICS) to companies that have achieved superior profitability by implementing unique strategies.

2011
Training
TRI Training Program with JICA
Launches a training program for physicians in Latin America, in collaboration with the Japan International Cooperation Agency (JICA).

Global Network
Terumo Yamaguchi Corporation
Opens a factory in Yamaguchi, its second to produce catheters after the Ashitaka Factory.
2012
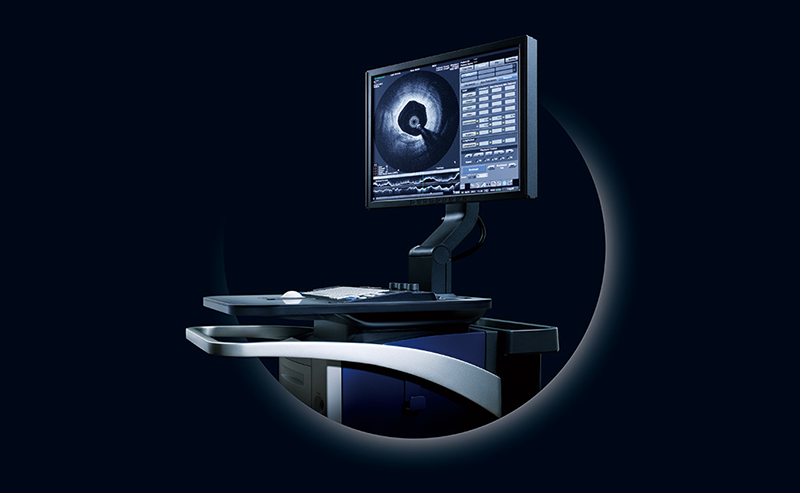
Product Launch
LUNAWAVE™ FastView™
Launches an Optical Frequency Domain Imaging (OFDI) product, thereby becoming able to support treatments with both IVUS and OFDI imaging systems.

2013
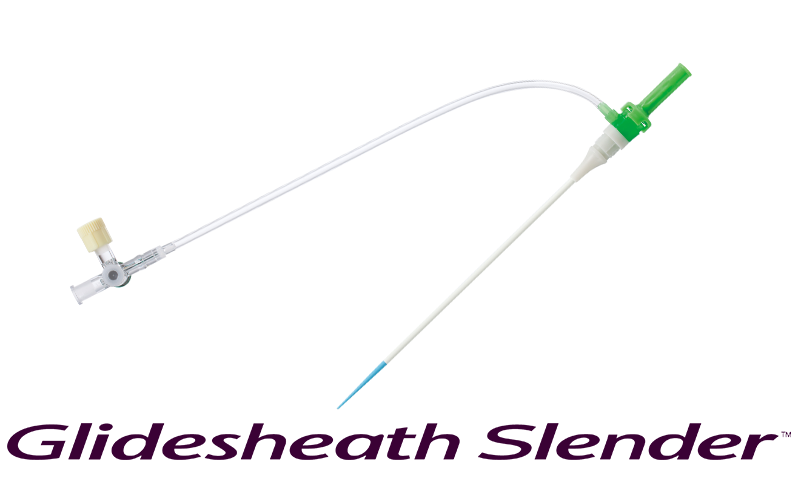
Product Launch
Glidesheath Slender™
Develops the Glidesheath Slender™, designed to help make treatment minimally invasive by having a thinner outside diameter while maintaining the necessary inner diameter.
2014
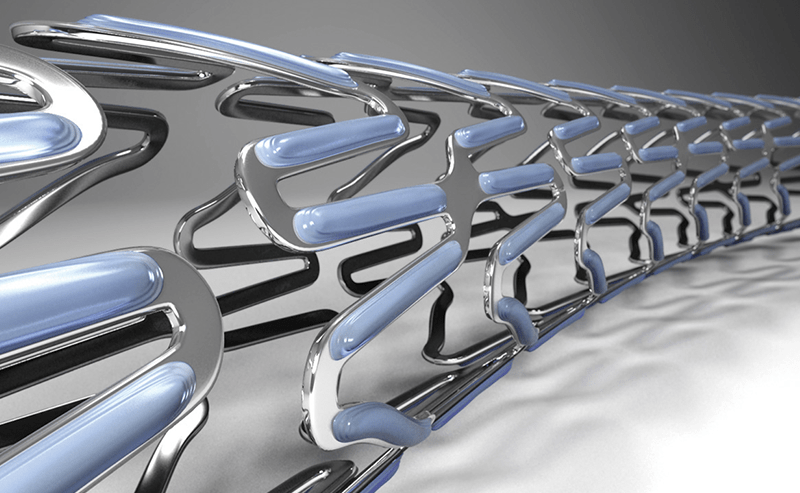
Product Launch
Ultimaster™
Launches Ultimaster™, a highly flexible sirolimus eluting coronary stent system and the successor to Nobori™.
2015
Organization
Terumo Interventional Systems
Changes the name of the Group's catheter company to Terumo Interventional Systems (TIS).

Brand
Standalone TIS Brand
With the change of name, also starts developing TIS as a standalone brand.
Training
Terumo Learning Edge™
Establishes an organization for conducting internal and external education and training.

2016
Global Network
Terumo and Quirem Medical Expand Strategic Alliance
Adds microspheres for radioembolization treatment to portfolio. (Completes acquisition of Quirem Medical in 2020.)

Global Network
Terumo Business Edge
Launches a new service in the US, offering solutions to help optimize performance and care delivery in hospital catheter labs.

2017
Product Launch
Angio-Seal™
Adds vascular closure devices to portfolio.

2018
Training
Collaboration with EuroPCR
Delivers eight workshop sessions using simulation-based learning for PCI complications through partnership between Terumo Learning Edge (TLE) and EuroPCR.

Brand
R2P™ system
Proposes the Radial to Peripheral (R2P™) concept, involving conducting peripheral intervention via the radial artery.

Brand
R.A.V.I. - Radial Access for Visceral Intervention
Similarly, starts promoting visceral intervention via the radial artery, calling it Radial Access for Visceral Intervention (R.A.V.I.).

Global Network
Acquisition of Essen Technology (Beijing) Co., Ltd.
Acquires Essen Technology with the aim of enhancing local sales and service delivery capabilities in China.

2020’s
2021
Global Network
Acquisition of Health Outcomes Science, Inc.
With the ePRISM digital platform, aims to further optimize patient prognoses and cost-effectiveness of care in the US.


