DISCOVERY 1TO3
Overview
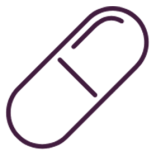
・DISCOVERY 1TO3 demonstrated rapid strut coverage of UltimasterTM DES, with the majority of strut coverage process taking place within the first month after stent implantation.
・The unique sequential OFDI analysis at 1, 2 and 3 months of treated lesions improves our insight into the embedding of novel metallic BP-DES.
Study Design
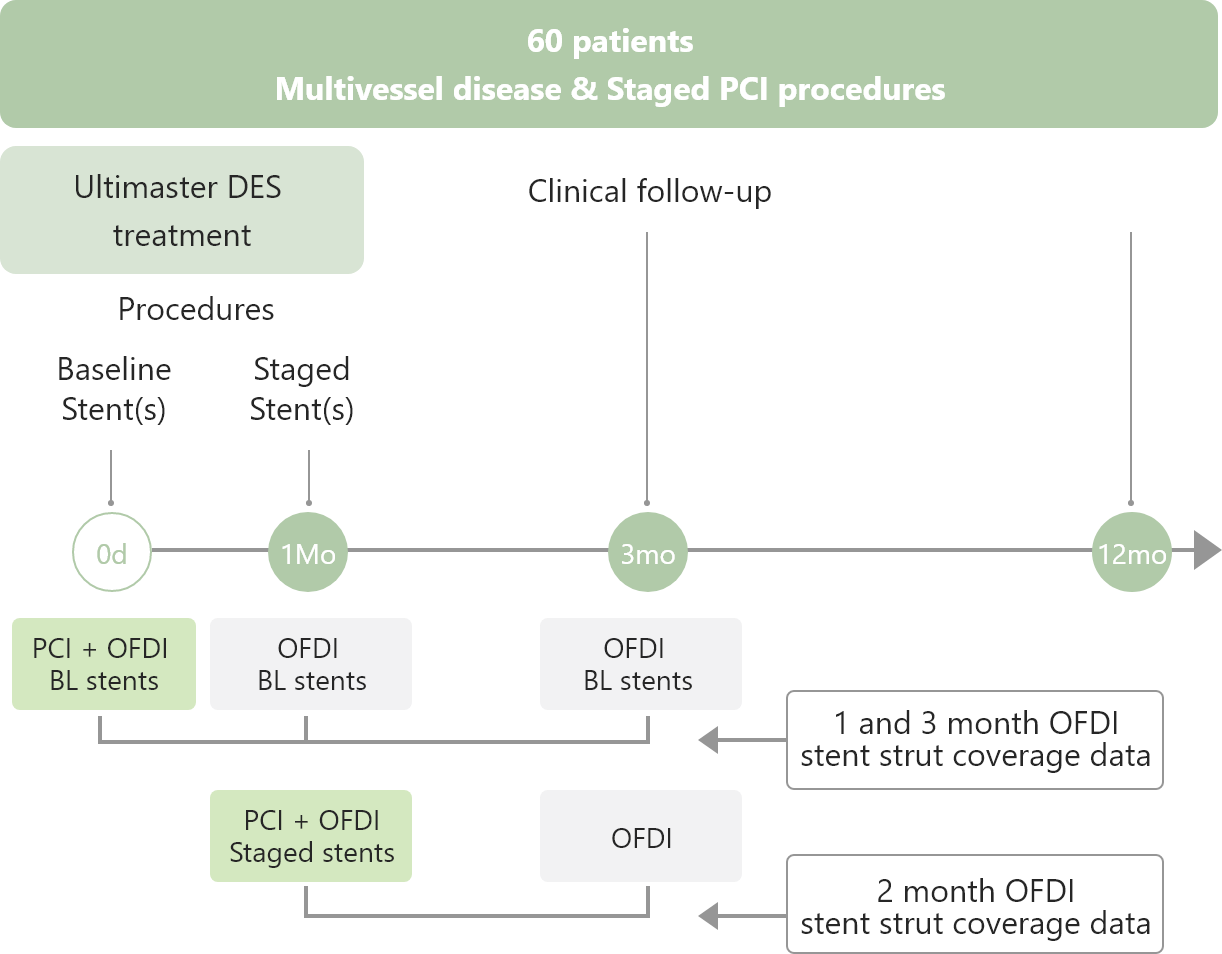
Study Outline
Patient background comparison with historical control
| Baseline Patient Characteristics | n=60 Patients* |
|---|---|
| Age, y (mean±SD) | 67.2+9.9 |
| Sex, males (%) | 73.3 |
| Diabetes mellitus (%) | 23.3 |
| Hypertension (%) | 63.3 |
| Dyslipidemia (%) | 67.8 |
| Family history of CAD (%) | 30.0 |
| Current smoker (%) | 13.6 |
| Previous PCI (%) | 26.7 |
| Previous CABG (%) | 3.3 |
| Previous MI (%) | 21.7 |
| Angina status (%) | |
|---|---|
| Stable patients | 56.7 |
| ACS | 36.7 |
| Moderate renal insufficiency GFR or MDRD=45-60), (%) | 20.0 |
| Baseline lesion characteristics* | |
|---|---|
| Vessels diseased | 2.2±0.5 |
| Lesions detected | 3.2±1.6 |
| Lesions treated-baseline | 1.4±0.6 |
| Lesions treated-staged procedure at 1 mo (37 patients) | 1.1±0.4 |
| Total lesions treated | 2.1±0.7 |
| Stents implanted per lesion | 1.2±0.4 |
| Implanted stent length per lesion, mm | 22.0±10.9 |
| Implanted stent length per patient, mm | 50.4±21.4 |
ACS indicates acute coronary syndrome.
*One patient withdrew consent.
*One patient withdrew consent.
Result
Clinical outcomes
Frequency of strut coveredge of combind both single and overlapped stents at 1,2, and 3 months.