Features and Types of Ultimaster™ Stent
Special Topic
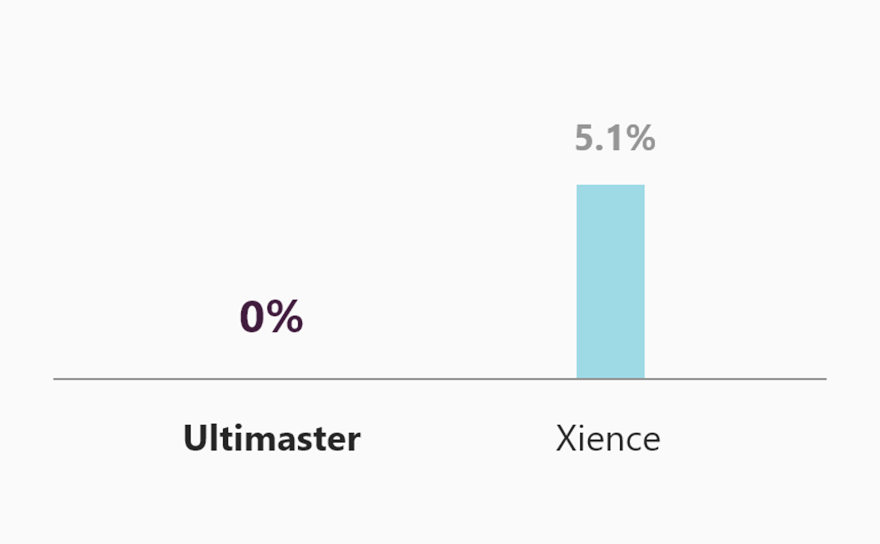
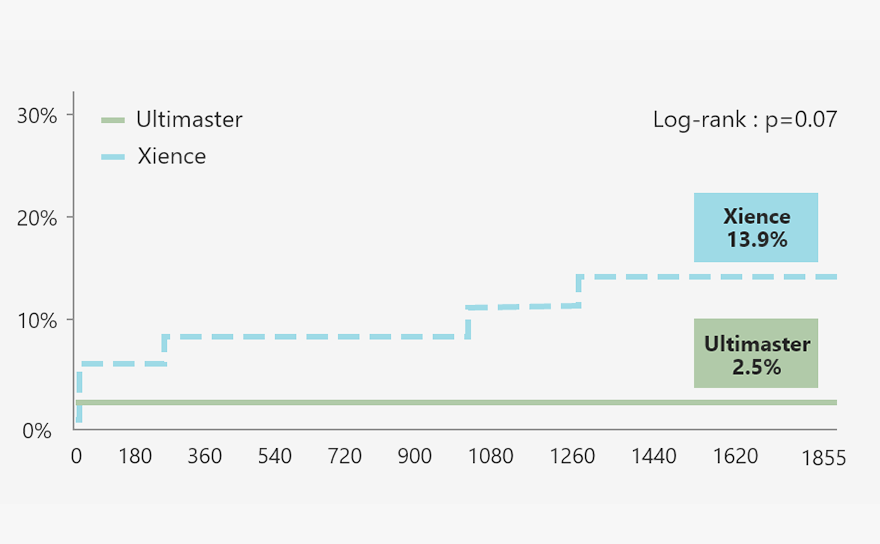
New insight from MASTER DAPT
MASTER DAPT Complex PCI sub-analysis presented at EuroPCR 2022. You can download it here.
MASTER DAPT result
Click here to download a summary of trial primary endpoints.
The most studied BP-DES, over 50,000 patients world wide1,2
Ultimaster™ has been studied worldwide by enrolling over 50,000 patients in Terumo sponsored trial and investigator sponsored research. The clinical programs have consistently shown favorable efficacy and safety of Ultimaster™ even in complex lesions.
| Number of patients | Design | Primary outcomes | Sub Study | |
|---|---|---|---|---|
| MASTER DAPT | 4300 | Investigator-initiated, randomized, short vs standard DAPT | NACE, MACCE, MCB at 11 months (12 months post-index PCI) | Enrolling, protocol published |
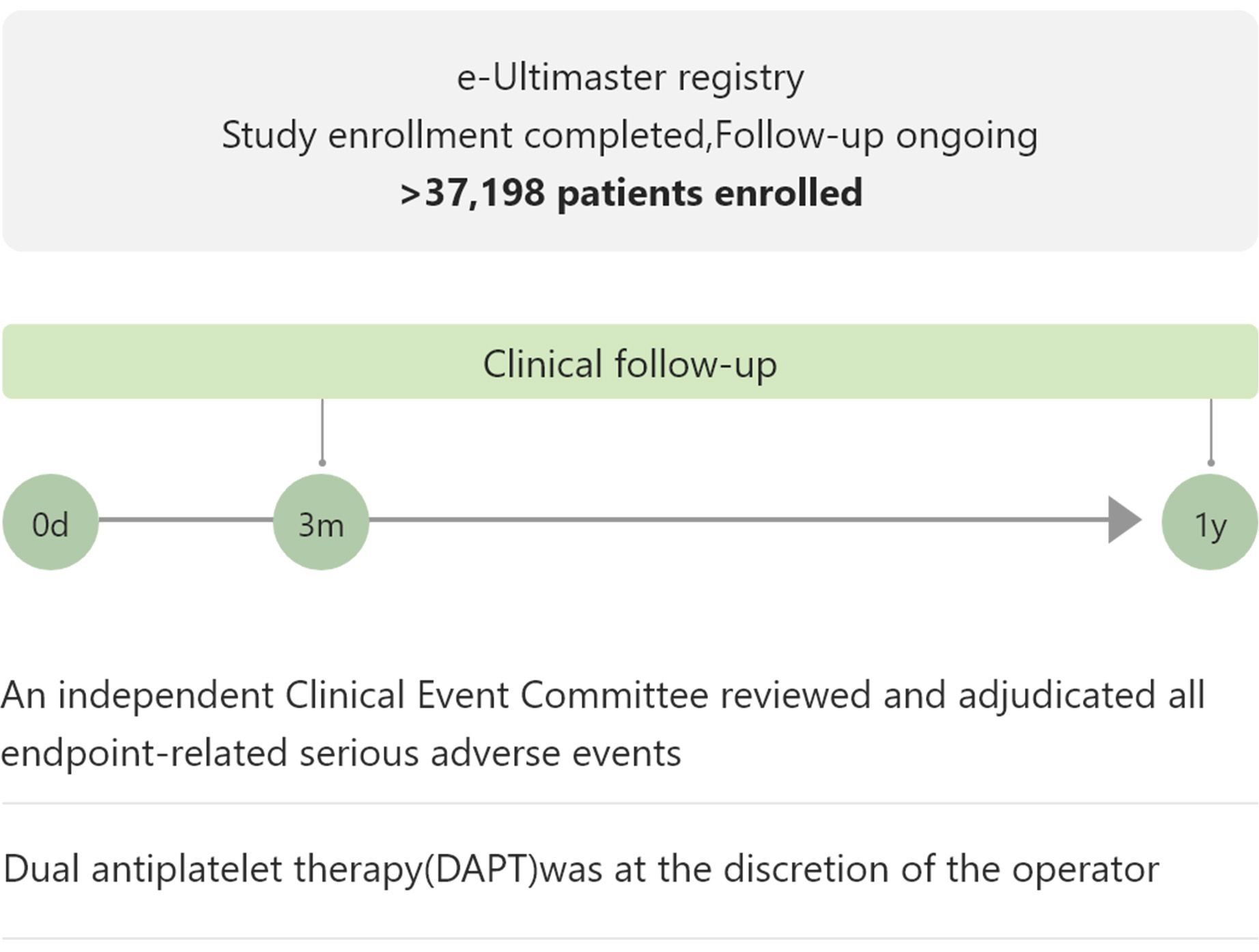
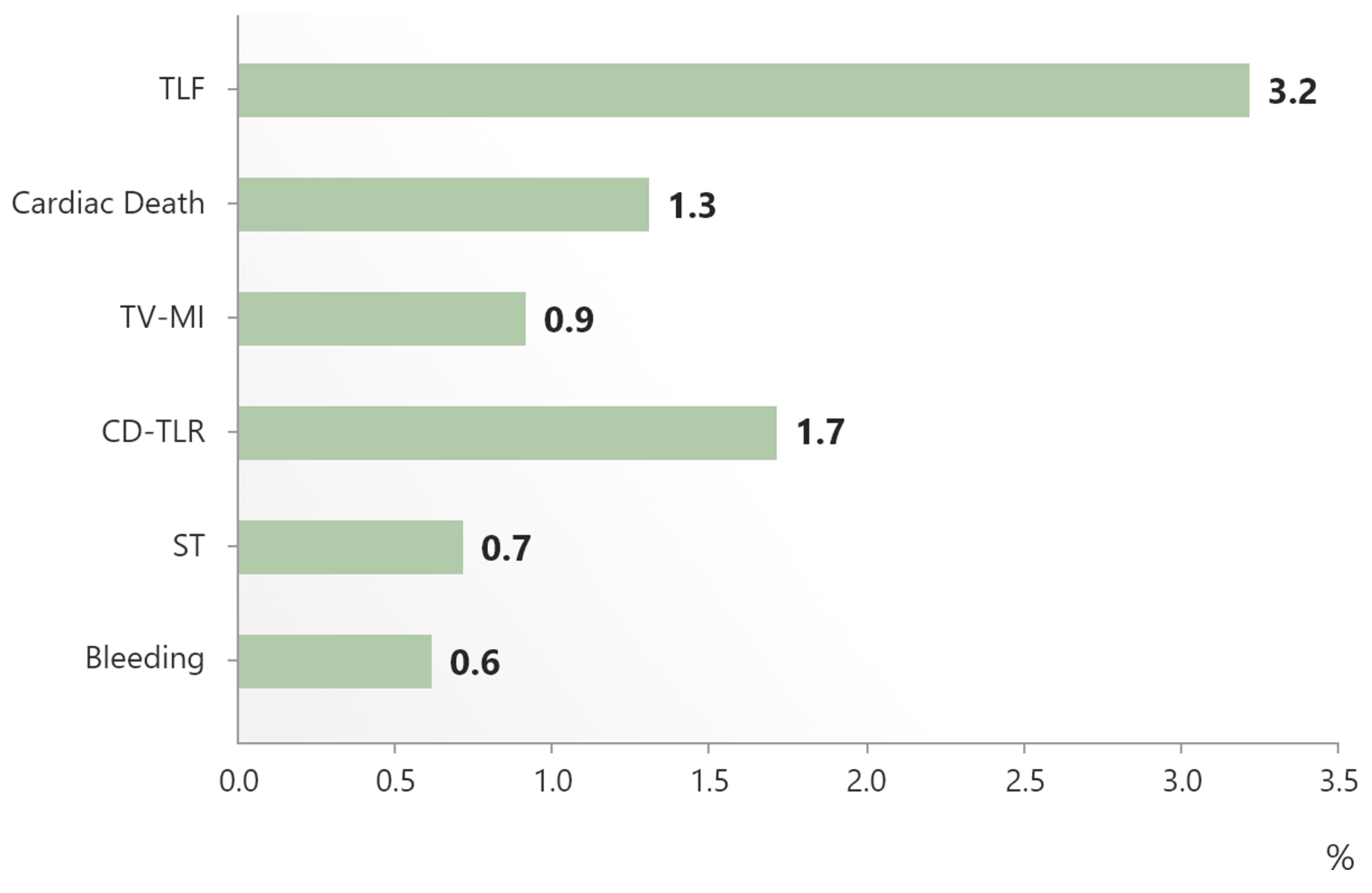
| e-Ultimaster | 37,198 | Single arm, all-comers, real-world use | TLF at 1 year | Completed and accepted for publication |
| CENTURY II | 1123 | Randomised 1:1 vs Xience | Freedom from TLF at 9 months | Published and completed |
| DISCOVERY 1TO3 | 60 | Single arm, patients with multivessel disease | OFDI strut coverage at 3 months | Published and completed |
| MODEL U-SES | 1,695 | Prospective multicenter, single-arm registry, Investigators initiated study | Composite endpoint of all-cause death, MI, stroke, ARC definite / probable stent thrombosis, and BARC 3 or 5 during 12months after stenting | Published and completed |
| SCAAR | 3,253 | Multicenter registry in Sweden | ST, Restenosis rate | Enrolling |
| SCC registry | 1,727 | Single arm, all-comers, single center study | TLR, TLF, MACE | Published and completed |
| ULISS | 1,660 | Single arm, all-comers, single center study | TLR, TLF, Composite endpoint of cardiac death, TV-MI | Published and completed |
| MASTER | 500 | Randomized 3:1 vs BMS in patients with STEMI | Safety at 1 month, efficacy at 6 months, safety and efficacy at 12 months | Published and completed |
| COLOR | 388 | Investigator-initiated, randomized complexed large-bore radial PCI trial | BARC bleeding or vascular complication related to access site | Enrolling |
| CENTURY | 105 | Single arm, first-in-man study | Late loss at 6 months | Published and completed |
| CENTURY JSV | 70 | Single arm, patients requiring 2.25 mm diameter stents | Freedom from MACE at 9 months | Published and completed |
| TCD-10023PK | 22 | Single arm, pharmaco kinetics | Sirolimus concentration in peripheral blood samples 28 days after Ultimaster implantation | Published and completed |
View more about clinical evidence
The biggest real-world BP-DES registry shows favorable clinical performance3
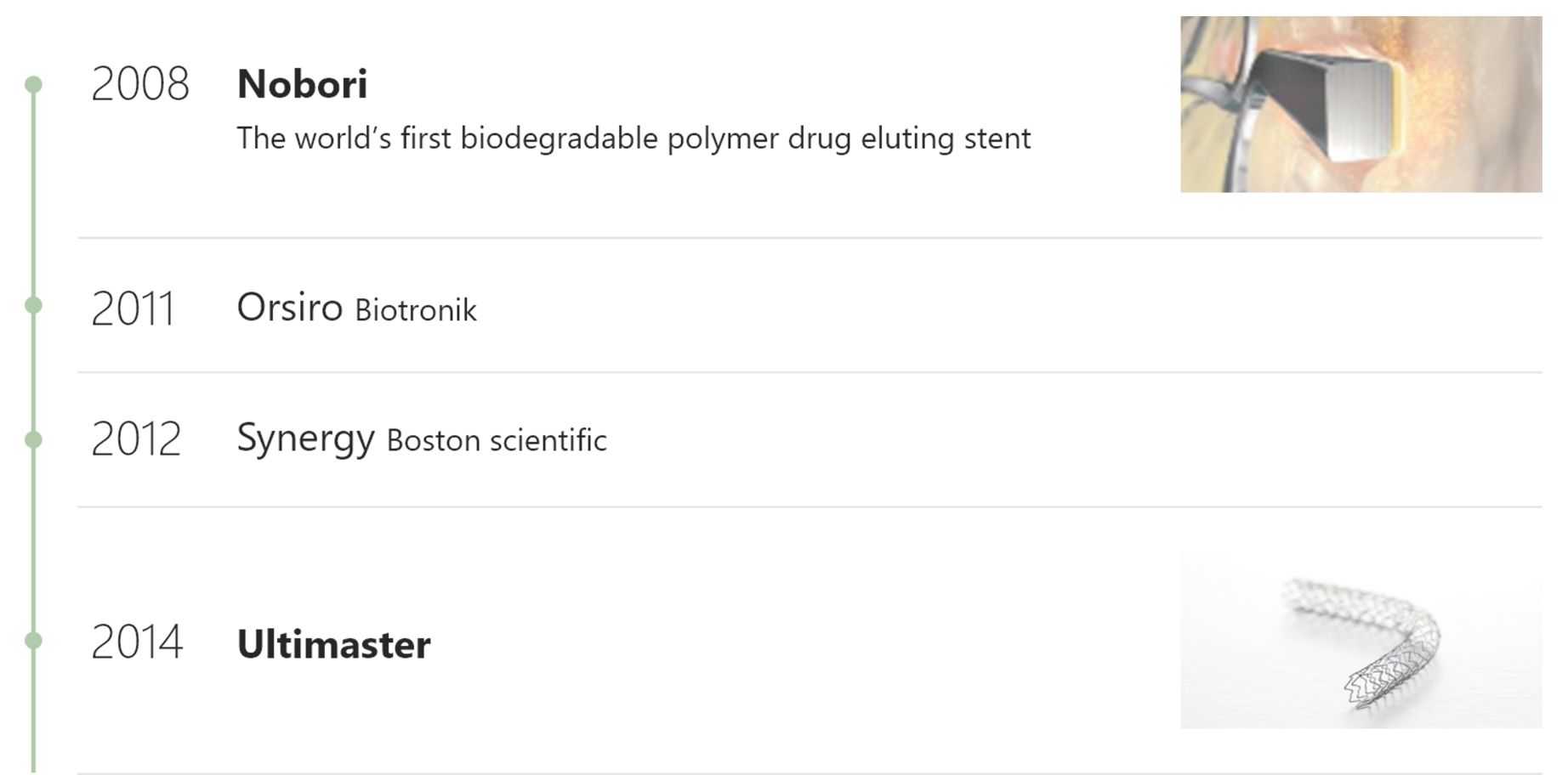
The Longest history BP-DES company4

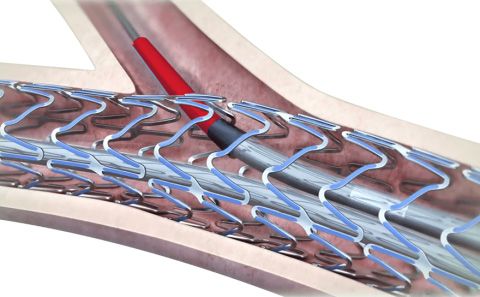
Good transmission force even when the guiding catheter does not provide sufficient support1
Ultimaster™ is designed to facilitate delivery for TRI. In TRI, guiding catheter sometime fails to provide sufficient back-up support, leading to guiding catheter disengagement in complex lesion treatment. Ultimaster™ supports not only delivery in challenging cases but also your thought for your patients.
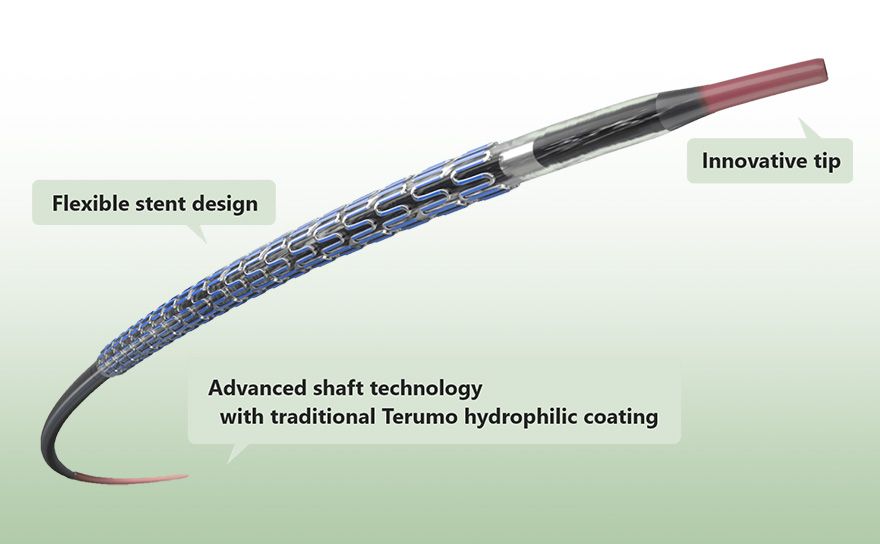
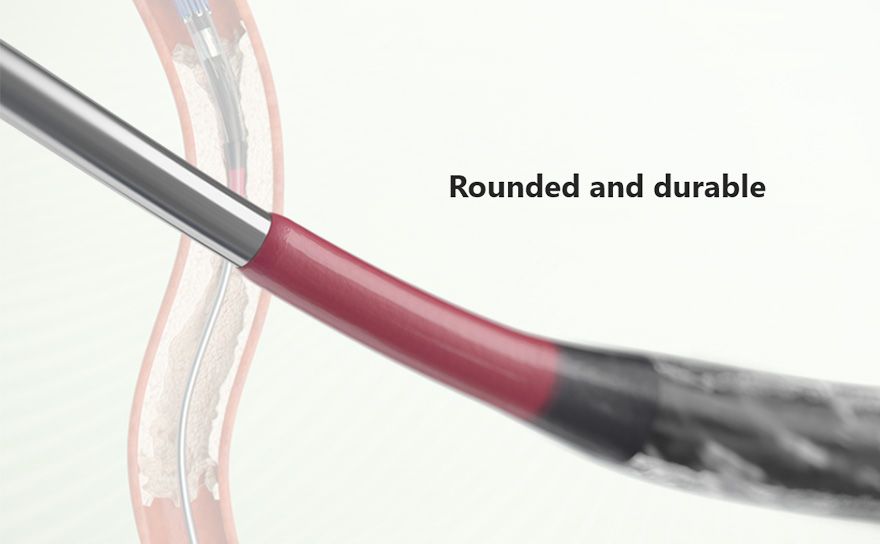
Advance shaft technology with traditional Terumo technology
Ultimaster™ was developed by applying both the advanced technology used for the Terumo PTCA balloon shaft and the traditional hydrophilic coating technology used for the Terumo guidewire. These technologies enhance pushability and trackability under challenging conditions such as TRI where back-up support from a guiding catheter is poor.


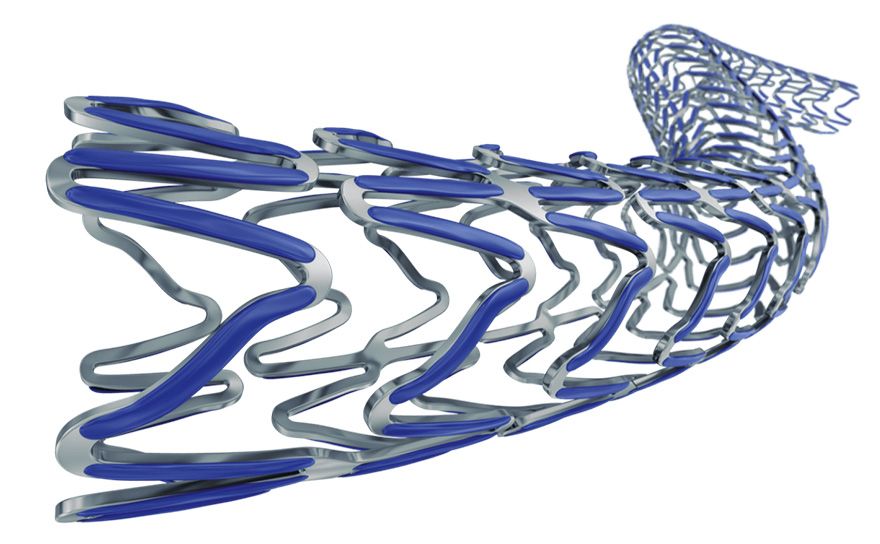
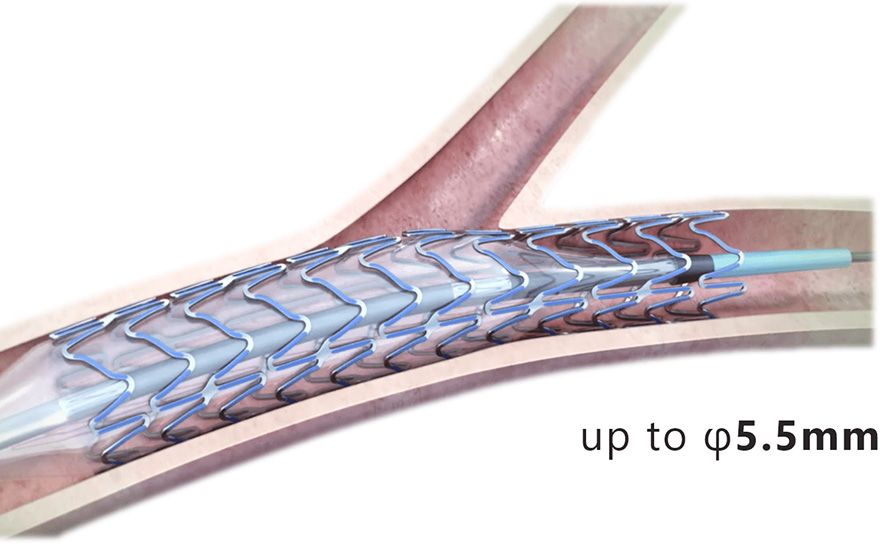
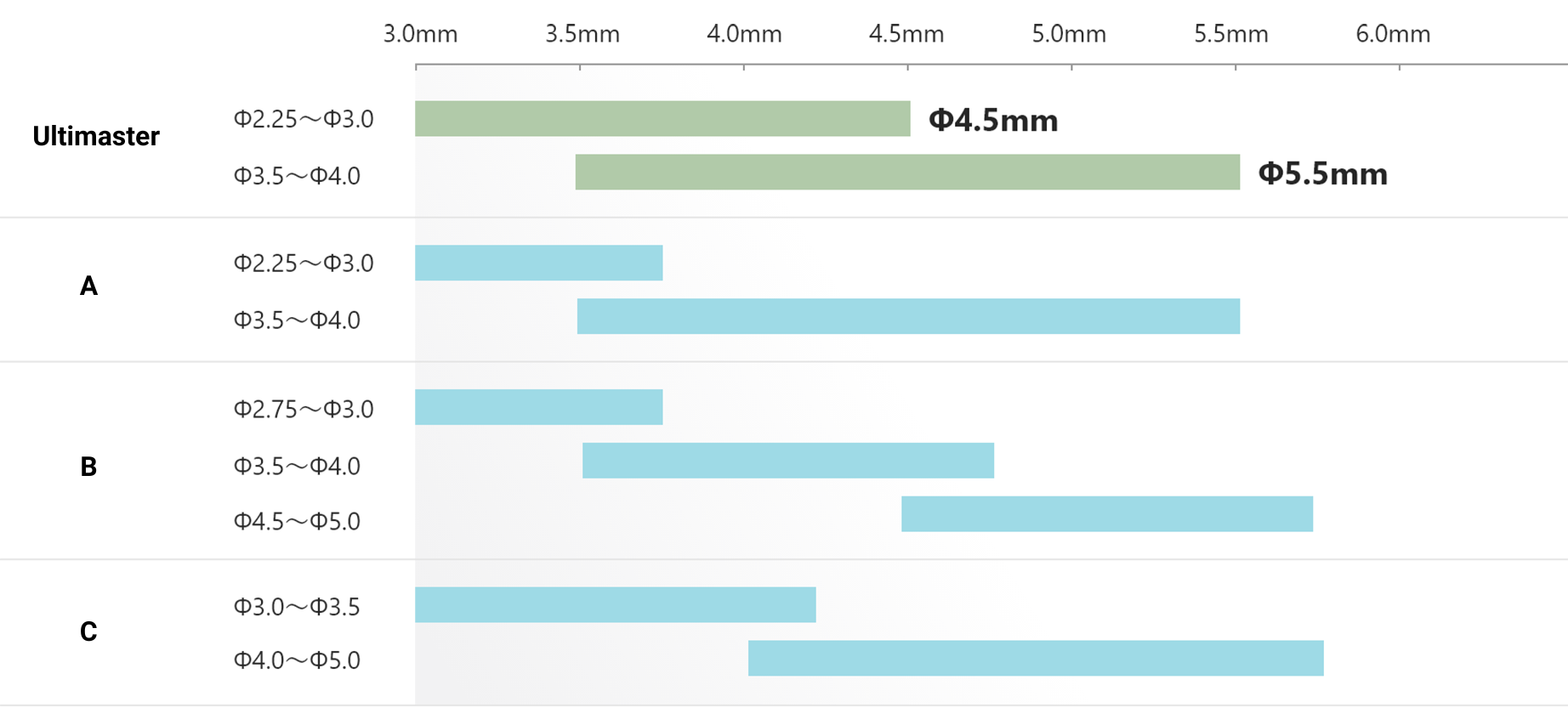
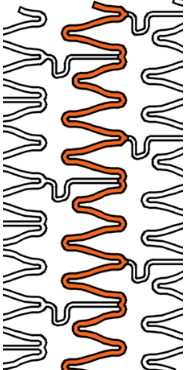
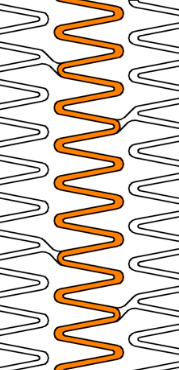
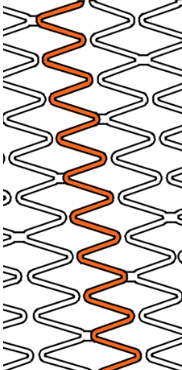
Wider over-expansion range
| Stent design forΦ4.0mm | Ultimaster™ | A | B | C |
|---|---|---|---|---|
| Stent Design | 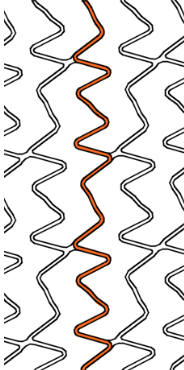
| 
| 
| 
|
| Measured total crown length | 21.5mm | 20.1mm | 19.8mm | 15.2mm |
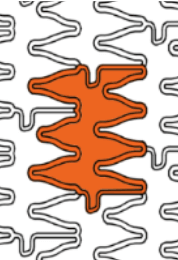
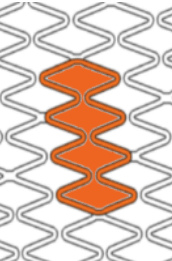
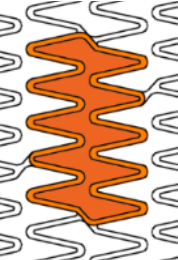
Smooth side branch access by the biggest open cell size design
| Stent design forΦ4.0mm | Ultimaster™ | A | B | C |
|---|---|---|---|---|
| Stent Design | 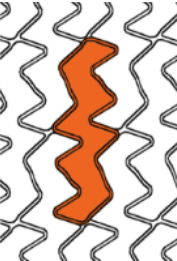
| 
| 
| 
|
| Measured total crown length | 21.5mm | 20.1mm | 19.8mm | 15.2mm |
| Maximum Cell Diameter | 6.8mm | 4.8mm | 5.7mm | 6.5mm |
| Maximum Cell Area | 36.3mm2 | 18.1mm2 | 25.7mm2 | 33.2mm2 |
Abluminal gradient coating technology ensuring polymer integrity
Our products are manufactured by paying attention to the details. There is no drug coating on the stent platform at which high stress is concentrated when stent is over-expanded. Ultimaster™ keeps polymer integrity even when the stent is expanded to the maximum diameter.





