MASTER DAPT Complex PCI sub-analysis


Overview
Investigator-initiated
global randomized study
global randomized study
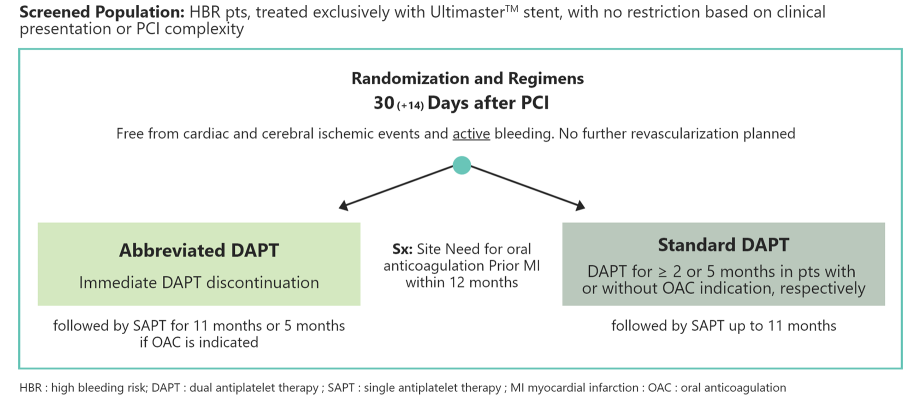
4,579 patients,
140 hospital worldwide, 30 countries
140 hospital worldwide, 30 countries
All-comers trial in
HBR patients after PCI
HBR patients after PCI
Abbreviated vs prolonged
DAPT in patients treated with
the Ultimaster™and Ultimaster™ Tansei™ DES
DAPT in patients
treated with the
Ultimaster™ and
Ultimaster™Tansei™
DES
treated with the
Ultimaster™ and
Ultimaster™Tansei™
DES
Primary Endpoint
- Net adverse clinical events (NACE) defined as a composite of death from any cause, myocardial infarction, stroke or major bleeding.
- Major adverse cardiac and cerebrovascular events (MACCE), defined as a composite of death from any cause, myocardial infarction, stroke or major bleeding.
- Major or clinically relevant non-major bleeding (MCB), defined as BARC 2, 3 or 5 bleeding.
Result
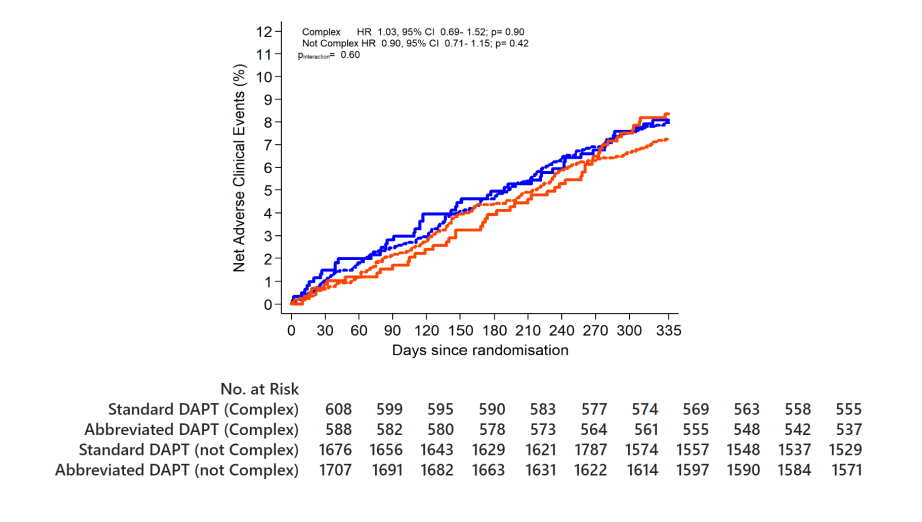
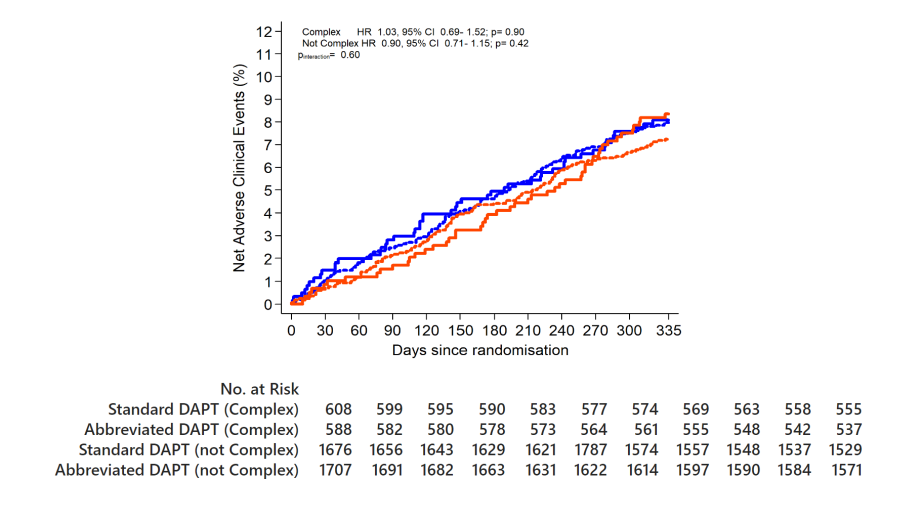
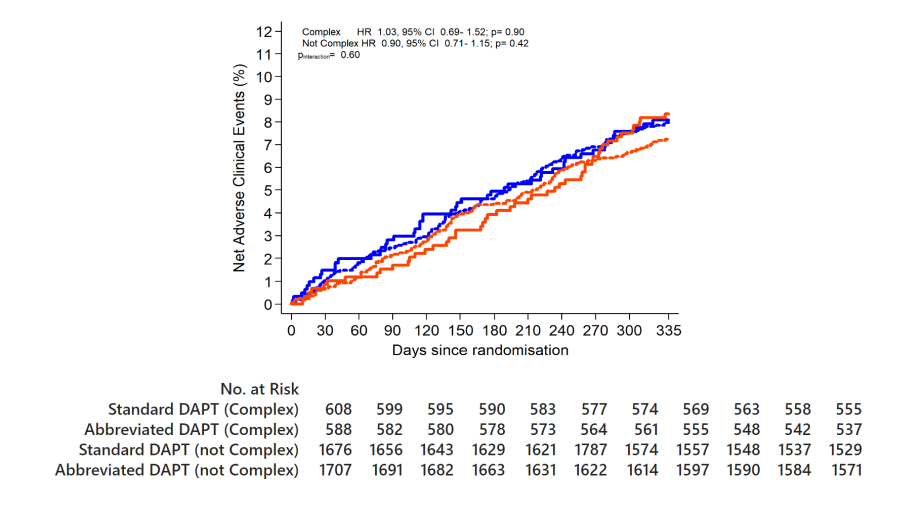
NACE
Abbreviated DAPT is non-inferior to standard DAPT in terms of NACE
MACCE
Abbreviated DAPT is non-inferior to standard DAPT in terms of MACCE
Complex HR 1.24, 95% CI 0.79-1.92; p=0.35
Not Complex HR 0.91, 95% CI 0.69- 1.21; p=0.52
Not Complex HR 0.91, 95% CI 0.69- 1.21; p=0.52
Conclusion
In HBR patients who had undergone implantation of Ultimaster™ stent, regardless of PCI-complexity, the discontinuation of DAPT at a median of 34 days compared with continuation of treatment for a median of 193 days after PCI was consistently associated with:
- Similar rates of net adverse clinical events(NACE) and major adverse cardiac or cerebral events(MACCE)
- A lower rate of major or clinically relevant nonmajor bleeding
Trial Limitations
- Open label study.
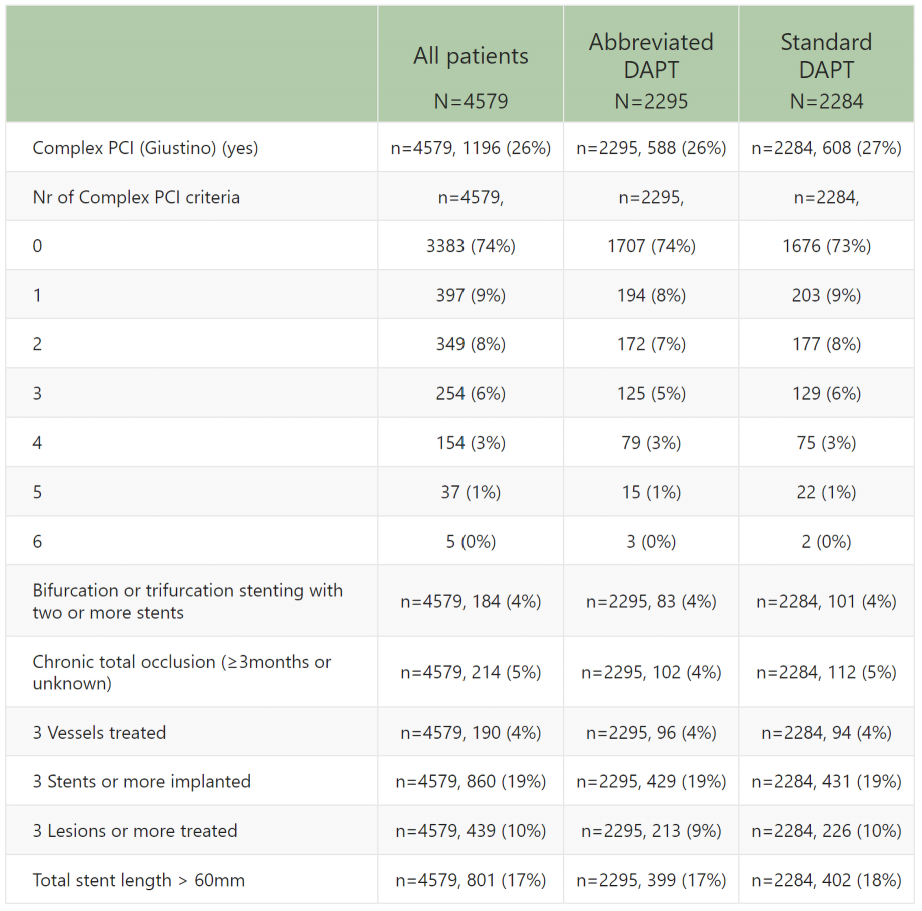
- Randomization was not stratified based on PCI complexity. The absence of a universally accepted definition for complex PCI is notable.
- The results may not apply to patients not treated with UltimasterTM biodegradable-polymer sirolimus eluting stents.
- The type of monotherapy after discontinuing dual antiplatelet therapy was at discretion of the treating physicians.
MASTER DAPT study is sponsored by the European Cardiovascular Research Institute (ECRI, Rotterdam, The Netherlands) and supported with a restricted research grant by Terumo Europe.