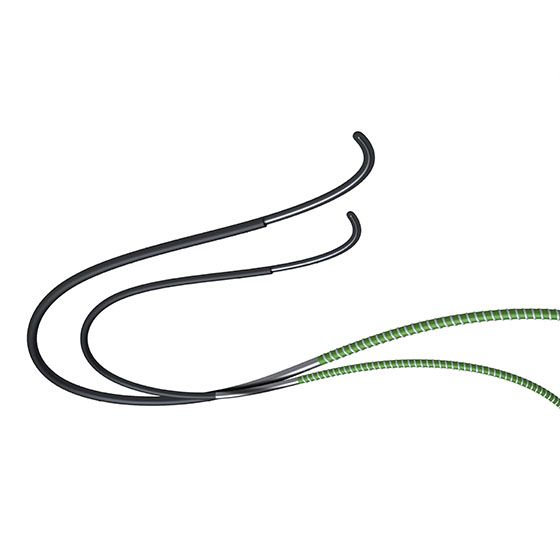
The combined use of Terumo’s three endovascular devices - Destination™, NaviCross™, Glidewire Advantage™ - aims to simplify procedures for better access and crossing in peripheral intervention. Learn about the latest initiatives and their unique perspectives, from three associates at the heart of these products.

Takashi Ito
Senior Engineer,
Ashitaka Factory
Exclusively worked on development of the peripheral support catheter, NaviCross. Extensively involved in development of a varied lineup of sizes and lengths. Recently, engaged in exploring value delivery for TLA* devices.

Kanako Furuta
Product Manager, Peripheral,
Global Marketing
Transitioned to marketing from sales.
As Product Manager, engaged in Glidewire Advantage development, and initiatives to enhance the overall deliver of value in peripheral treatment.

Mitsuki Hoshi
Research Engineer,
Ashitaka Factory
Assigned to NaviCross design modification project on joining Terumo. Interacting with healthcare professionals, applies insights to new product design and development of hands-on models.

Kanako Furuta (KF): While we at Terumo have historically focused on the cardiology sector, we see peripheral intervention as an area where we can genuinely make a difference.
- D.N.A. - Destination, NaviCross, Glidewire Advantage
KF: D.N.A. is an abbreviation inspired by our trio of endovascular devices: Destination (D), NaviCross (N), and Glidewire Advantage (A). As cases of complex peripheral lesions continue to rise, we see our D.N.A. solution as a way to bring together the unique benefits of each device, combining their strengths to effectively improve success rates in lesion crossing.
Takashi Ito (TI): We’d begun designing an aorta-iliac model to more effectively convey the tangible benefits of our D.N.A. solution. Initially, the model was conceived as a training tool, but as we moved forward with the design, we began to see it had potential as a hands-on model. So, we shifted gears and began creating a hands-on model that could be used for customer-facing demonstrations.
The biggest challenge was creating a model that authentically recreated lesions. We’d test, make tiny adjustments—no more than 0.1 mm—test again, adjust again, and on it went. Finally, after six months of trial and error we had a hands-on model we were genuinely happy with. Having Mitsuki in the team was a huge boost. Her previous experience with designing a hands-on model was invaluable in seeing this project through to completion.
Mitsuki Hoshi (MH): Previously, I’d created a hands-on model for a different device. The manufacturer’s model came up short in highlighting the value and advantages of our device, and through a process of trial and error, I was able to make a model that could. I also wanted our overseas associates to use it, so it had to be sturdy enough to safely ship and easy to assemble. And, of course it was crucial that the model could reliably reproduce results no matter what. A lot of thought went into the entire design process.



- Which of the three personalities, Imaginative Ideas, Attention to Details, Never Give Up, resonates with you as an individual?
"Never Give Up"

"Imaginative Ideas"

"Imaginative Ideas"
TI: Imaginative Ideas for me too. When you’re faced with a tough situation, any idea, big or small, can spark something new. Coming up with fresh ideas and making them happen takes a lot of energy. But making the impossible possible is what keeps me moving forward. And doing something that no one else can is its own special sense of achievement. When an idea pops into my head, something I want to try out, I can’t wait for the next day to come and start working on it.

*TLA: Therapeutic Lesion Access
*Information accurate as of October 2023.
Other Stories

Discover the stories hidden in our exploration and challenges - how we push the boundaries in access devices.

Less-invasive solution for interventional oncology - learn how we push the boundaries in introducing RADIFOCUS™ Glidecath™, the first Terumo device for radial access to visceral.

Passion across three generations - engineers of Ultimaster™ talk about how we push the boundaries in developing each new generation of DES.