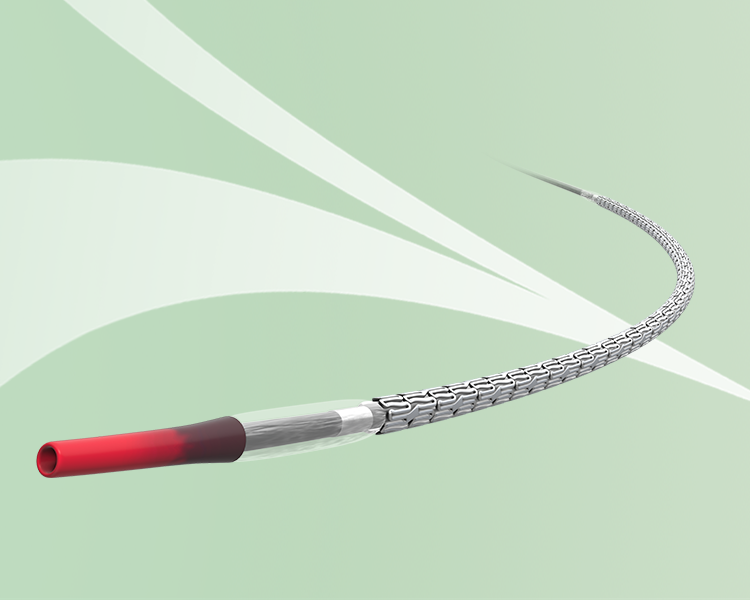
Since Terumo launched Japan’s first DES in 2014, Ultimaster™ has continued to evolve and gone through various transformations. The 3rd generation, Ultimaster Nagomi™, was released in 2022. Learn from three of our engineers involved in the development of Ultimaster™, Ultimaster™ Tansei™, and Ultimaster Nagomi™.

Kazuhiro Maruyama
Senior Engineer, Ashitaka Factory
After joining Terumo, worked in TIS R&D, focusing on the development of coating technology for stents. Established the gradient coating technique used on Ultimaster™. Currently engaged in production optimization for DES at the Ashitaka factory.

Ryota Ikeuchi
Senior Engineer, Ashitaka Factory
Involved in development of Ultimaster™ series since joining Terumo. Led collaboration with related departments to bring Ultimaster™ Tansei™ to market. Continues to be actively engaged in understanding needs and challenges in the field.

Ryosuke Ueda
Associate Product Manager, Cardiology, Global marketing
As part of R&D, joined the initial development team for Ultimaster Nagomi™. Currently promoting global release of the same product as part of the global marketing team.
- Abluminal gradient coating is a feature that has persisted across all Ultimaster™ generations. What challenges did you face in developing this?
Kazuhiro Maruyama (KM): Our starting point was Nobori™, Ultimaster™’s predecessor, a DES whose coating had been developed in-house. This was where the initial concept of abluminal coating began, and we carried it into Ultimaster™’s development.
But it wasn’t all smooth sailing. During development, the coating layer kept peeling away from the stent during expansion. This scenario posed a risk of distal embolization—a potentially dangerous situation for patients. We needed to come up with a solution.
The lightbulb moment came when we hit on the idea of eliminating the drug from the area that kept peeling away. But coming up with the idea was the easy part.
Stents are miniscule; each one mere millimeters in length, and the metallic parts only 0.01 mm in width. Creating a stable and safe method to selectively coat parts of such a tiny object was intensely demanding.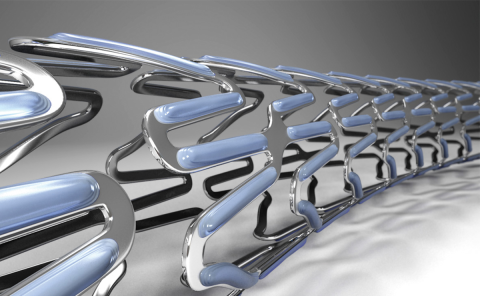
Trials involved different levels of adhesiveness, and we undertook multiple adjustments to the coating machine. Plus, ensuring that the coating speed was optimized for mass production added another layer of difficulty. There was also the issue of design. When we started developing the coating technology, the design of the stent was not finalized. Every tweak in the design necessitated an adjustment in the technology. Taking the project from concept to application was incredibly challenging.
But we always knew that, once perfected, this technique would enable us to provide profound value: a stent capable of ensuring patient comfort across every vessel. And this belief fueled our perseverance.
- Four years after the launch of Ultimaster™, Ultimaster™ Tansei™ was brought to market. What were the primary considerations during its development?
One of the main areas we focused on in Tansei™ was improving deliverability to distal arteries. But we knew from our experience with Ultimaster™’s rollout that it wouldn’t be enough to explain the specs. That alone would not communicate the benefit well enough to build trust among healthcare professionals. We needed to try something else.
So we innovated our communication approach by introducing a hands-on model to demonstrate in a more tangible way the improvements we’d implemented in Tansei™. This method, which has become standard now, was pioneered by Tansei™ and gave professionals a more experiential understanding of the product.
Listening to our customers also highlighted the necessity for speed and bringing improved product to medical settings as soon as possible. Time to market became a pivotal consideration in development and consequently, we decided to defer modifications to the stent design until Ultimaster Nagomi™. I’m aware that many developers believe in achieving full optimal potential in development, regardless of timeframe. However, I lean towards prioritizing the wants and needs of the customer in the present moment.
In this case, demand was shifting toward a swift rollout of an improved version. While there were many conditions to adhere to, when it all came together and Tansei™ was finally launched, there was a great sense of accomplishment.
- What challenges did you face when developing the 3rd generation Ultimaster™, Ultimaster Nagomi™?
Ryosuke Ueda (RU): With Ultimaster Nagomi™, we were presented with major challenges in two different areas: product development and regulatory affairs.
When it comes to product development, we wanted to modify the design of the stent without changing the drug, polymer, or coating technology. The drug was at the foundation of our clinical data. Keeping this consistent was paramount to ensure that healthcare professionals could seamlessly transition to the new model with confidence.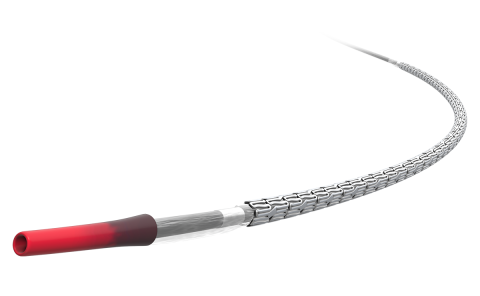
However, this was no easy task. Extending the length of the stent complicated the creating of a stable drug coating. A longer or slimmer stent typically had a negative impact on production. Creating a design that was compatible with the current coating technology and conducive to a stable supply proved to be an incredibly challenging puzzle to solve.

Our persistence bore fruit, enabling us to introduce Ultimaster Nagomi™ to customers worldwide.
- Development in the medical field often takes longer when compared to other consumer goods. Were there instances where your confidence wavered?

RI: Developing stents is a marathon, not a sprint. And unforeseen shifts in the environment can mean a change in course, sometimes midway through a project. It can be tough when that happens, and not everyone will be happy with it, but that’s why it’s so important to keep in mind the goal that we're all trying to achieve.
RU: As a developer, a sudden change in direction does make you go “you’ve gotta be kidding?”! At these times, we regroup and reaffirm our collective goal, and have honest and frank discussions to find the best direction to go in.
RI: Introducing a policy shift mid-project is bound to be met with some resistance. But as long as everyone can understand the logic behind it, we can refocus our energy, pull together and work on getting to the finish line.
KM: Trying something new is always going to be terrifying. I’ve experienced self-doubt more than once, and had the value of my work questioned by others. Venturing into new territory will always bring a mixture of pros and cons. The key is to be confident that the pros make it worthwhile. As engineers, we can integrate value into a product. And that’s why we have to stand firm and believe in the value of what we do.
And then I remember talking to my manager. He had a suggestion, but when I heard it, I started explaining all the reasons why it wouldn’t work, laying out every obstacle in the way. Then he said something that completely changed my mindset. “You’re an engineer, right? It’s not your job to list reasons why it won’t work —you should be considering how it can work. Stop rationalizing impossibilities, and start focusing on finding solutions.” It resonated with me as an engineer, but I think it’s something that rings true for any situation.
This gave me the inspiration I needed, and it was after that conversation that we finally landed on a coating idea that worked. The solution was something that looked theoretically unstable, but in practice worked. To be honest, nothing about this process was easy, but it made me realize how crucial it is to have a solution-orientated mindset that can flip the impossible to possible.
- What are your goals for the future?
"Bridge the gap between development and marketing"

"More global standpoint and deliver value"

"Valuing the tangible product is going to be key"
KM: Right now, I’m focused on homogenizing and streamlining production methods, which is very different to creating something from nothing. There’s a lot to gain from this experience and I hope that in the future I get to work on a new development project where I can put this knowledge to use. I’m an engineer at heart and getting hands-on with products is what I thrive on.
As AI and digital technologies become more prevalent, valuing the tangible product side is going to be key to adapting to these changes. And, I hope that my contributions will make a meaningful impact, in one way or another.

*Information accurate as of October 2023.
Other stories

Discover the stories hidden in our exploration and challenges - how we push the boundaries in access devices.

Less-invasive solution for interventional oncology - learn how we push the boundaries in introducing RADIFOCUS™ Glidecath™, the first Terumo device for radial access to visceral.