R2V™ - Radial to Visceral intervention expands the benefits of radial access to the whole body. Our engineers and a marketing lead talk about the challenging process to launch RADIFOCUS™ Glidecath™, the first device in this product portfolio.

Kengo Mitsukiyo
Director, Interventional Oncology,
Global Marketing
After joining the company, worked in sales for coronary intervention, gaining a deeper understanding of clinical setting. Later transferred to company headquarters. After a four-year posting in India, is now leading initiatives in the new domain of interventional oncology.

Ryo Okamura
Research Leader,
Technical Coordinate Office
Engaged in access devices for around 15 years. Involved in a wide range of activities, from concept planning and design to mass production and post-launch follow up. As part of Technical Coordinate Office R&D, works on promoting global R&D collaboration and joint development.

Mitsuteru Yasunaga
Senior Engineer, Ashitaka factory
After joining the company, engaged in new product development including R2P™ SlenGuide™ and the R2V™ shape for Glidecath™. Played a pivotal role in realizing mass production for Glidecath™ MG1/MG2 curve shapes. Currently involved in developing next-generation access devices for a new field.
- What sparked the concept for R2V™?
![]()
However, despite awareness around the benefits such as early hospital discharge and minimal invasiveness, there was a severe lack of dedicated devices on the market.
We develop devices for liver cancer treatment, and for the treatment of benign tumors—uterine fibroid embolization, prostrate artery embolization, and the like—and the inspiration came from a desire to find less invasive ways to treat these cases. We saw a need for a device that could treat patients in a minimally invasive way, and began exploring the possibilities.
Ryo Okamura (RO): Shortly after wrapping up Glidesheath Slender™, the marketing team approached me to begin development on a radial access device for interventional oncology. I began with a feasibility study with my then manager, a person whom I held in great esteem and knew could bring a global perspective to the project. Later, Mitsuteru joined the project, helping propel us forward and bringing us to launch. And that led us to the product we have now, Glidecath™’s R.A.V.I. MG1・MG2 curve shapes.
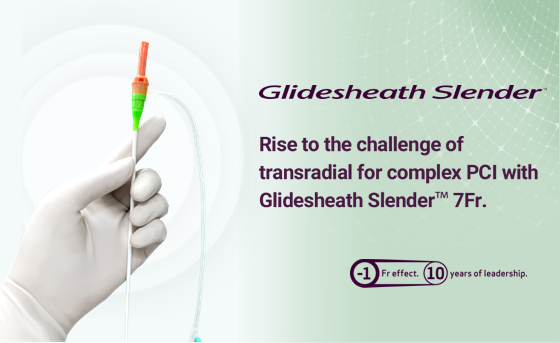
- Glidesheath Slender™ has been an essential element of Terumo’s commitment to radial access. Can you talk us through its evolution and the challenges you faced?
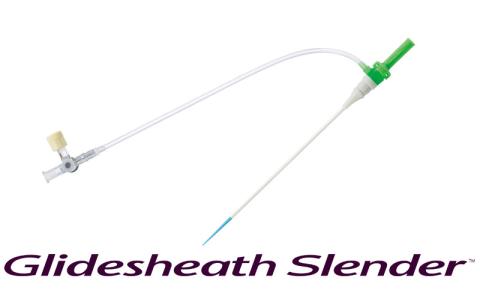
We understood the need, but addressing that need was slightly more complicated. We made numerous deliberations over the sheath material: should we use a new material? Or should we try to work with what we have and push it to its limit? In the end, we decided to stick with the same sheath material and produce the slimmest possible design, bringing it the very limit of durability.
Yet, this presented a challenge for production. Glidesheath Slender™ is made on the same production line as other Terumo sheaths, but due to its extreme thinness, it could easily break if handled in the same way by the production staff. Test runs showed that the conventional production process would crush or dent the sheath, and the initial yield rate was less than 10%.
We decided to revisit our entire production approach. From altering processes to refining training methods and adjusting equipment, we made multiple modifications until finally we were able to launch it to market. There were many times when I was so focused on my work, tweaking the equipment, that I’d lose track of time and end up working late into the night.
For me, engineering is about having the passion to deliver a device to the customers. Innovation always has meaning, but true value lies in creating something that aligns with the customer’s needs and successfully introducing it to the market. In those days, I was still learning on the job, and there was a lot I didn’t know, but this conviction helped me see the project through to completion.
- What did the process for developing dedicated products for R2V™ involve?

MY: Building on Ryo’s foundational design, my main role was to determine the exact specifications, and figure out how to integrate these into the production process.
- As Terumo already an angiographic catheter, Glidecath™, on the market, designing a new curve seems like a straightforward process. Was it?

For the catheter to perform as required, we had to bend the catheter by a certain amount to get the desired shape, but if bent too much, it would complicate the production process, leading to a fall in productivity.
Even if we made the curve using the same method as other Glidecath™ curve shape, we would end up with a new curve shape that did not necessarily achieve the performance we were aiming for. We looked at modifying the production conditions, adding new ones, and even considered revising the design. Deciding what to prioritize and where to compromise meant that it took quite some time to find a solution.
I don’t claim to have a stronger work ethic than anyone else, but I do believe that a job doing is worth doing well, and I always put my all into any project. It was my job not just to launch the device, but to deliver to market a device that met the needs of those in clinical settings, and the needs of our production team. The associates in production were very co-operative, and it’s thanks to their collaboration that we were able to successfully realize the efficient production of the device.
- Kengo, while you are currently leading the concept for R2V™, your beginnings are in the coronary field. How different is interventional oncology to your original area?

From the perspective of radial access, the level of adoption varies from country to country. We’ve designed the MG1 curve shape for liver conditions, and the MG2 curve shape for use with uterine fibroid embolization and prostrate artery embolization. I’d like to contribute further to the broader adoption of minimally invasive treatments through device development and training.
- For both you and Terumo, interventional oncology is a relatively new field. What drives you to venture into new areas?
KM: My four years in India taught me how important it is to keep an open mind when working, and not to be held down by any kind of preconceptions of “normal”. I tend not to dwell on past obstacles, so it’s easy for me to move on to the next challenge, and the prospect of entering unchartered territories is something that always appeals to me.
- Each of you have navigated a distinct career path. How do you see it progressing?
MY: Since joining the company, I’ve been privileged to contribute to new product developments, including devices such as R2P™ SlenGuide™, MG1/MG2 and YASHIRO Radial shape. Right now, I’m exploring device development in a new area. A call was put out for team members, I applied, and was accepted. The unique challenge of exploring a new field is something that appeals to me. In the future, I’d like to become an engineer with a global imprint. There’s a difference between the technology and talents of R&D at Ashitaka and our overseas bases, and I’d like to experience these first hand and add to my own skillset.
RO: For the past few years, I’ve been working at Technical Coordinate Office R&D, charged with the job of bringing together all our different technologies scattered around the globe—not only within TIS, but at TMCS¹ and TBCT² too. With each location independently progressing their own developments, the aim is to strengthen connections, enabling us to share technologies and enhance Terumo's overall technological development.
Still, my heart is with catheter development at Ashitaka factory, and I hope that one day I can use my current experience and apply it to benefit Ashitaka.
KM: Embolization and interventional oncology are areas witnessing rapid growth across the US and Europe. One way I can make meaningful contributions to this area is by fostering a team culture that embraces setbacks. I want my team to take on challenges without fear of failure, and I’m willing to take the responsibility for any mistakes that are made. Exposing my team members to a variety of experiences will make them into leaders who can, in turn, contribute their talent to the field.
*Information accurate as of October 2023.
Other stories

Discover the stories hidden in our exploration and challenges - how we push the boundaries in access devices.

Passion across three generations - engineers of Ultimaster™ talk about how we push the boundaries in developing each new generation of DES.