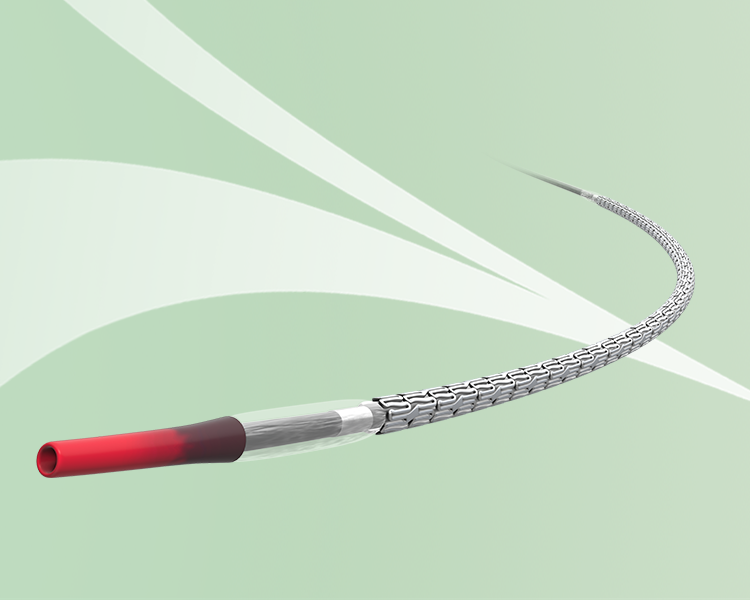
Navigate the complexities of every procedure for optimal outcomes for/with every patient
You can tackle any procedure with ease, adapting and conforming to the length and contours of vessels. Even in the most challenging anatomy, smooth trackability and reliable overexpansion allow for excellent maneuverability. Ultimaster Nagomi provides the versatility and flexibility you need to deliver the best possible results.1-3
As the newest solution in the Ultimaster™ product range — now with the largest size line-up — we build on its clinical legacy and expertise, adding a new level of innovation to your practice.1-3
Go anywhere with Ultimaster Nagomi™.
Improved Stent Design4,5
- 3 platforms specifically designed to meet the needs of each vessel size4,5
- Strut & band width shortened to help increase flexibility (for Ø2.00mm to Ø2.50mm stents)4-6
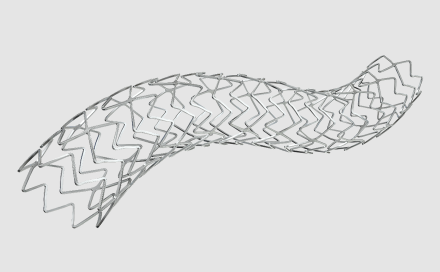
New 10-crown design in the large vessel :
to ensure better apposition even in large bifurcations, including left main trunk4,5,7
allows a better expansion capability with a uniform vessel coverage compared to an 8-crown design4,5,8,9
Larger Size Line-up11
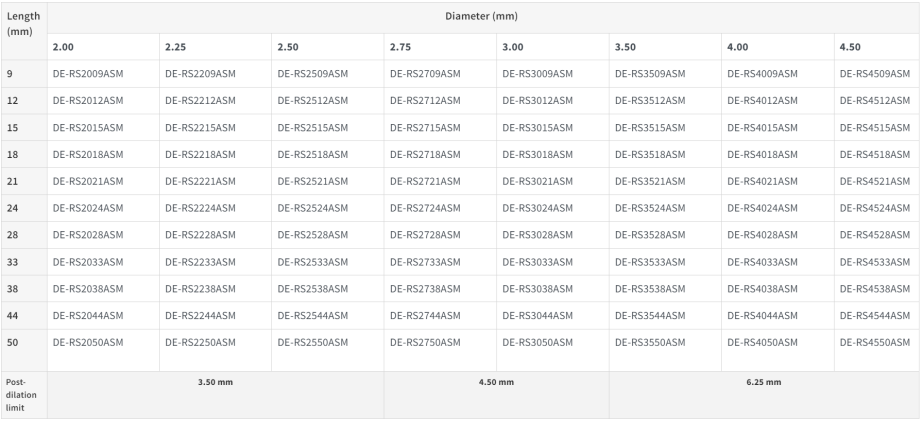
- The largest size line-up in the Ultimaster Family, from Φ2.00 to Φ4.50mm and 9 to 50mm length to treat a broader range of lesions4,5,8
New 44 & 50m lengths, for optimal sizing in long lesions4,5
Greater Overexpansion Capability11
- 10-crown design for a better expansion in large vessels4,5,8
- Optimized overexpansion capability, up to 6.25 mm (for Ø3.50mm to Ø4.50mm stents)8
Excellent Deliverability11
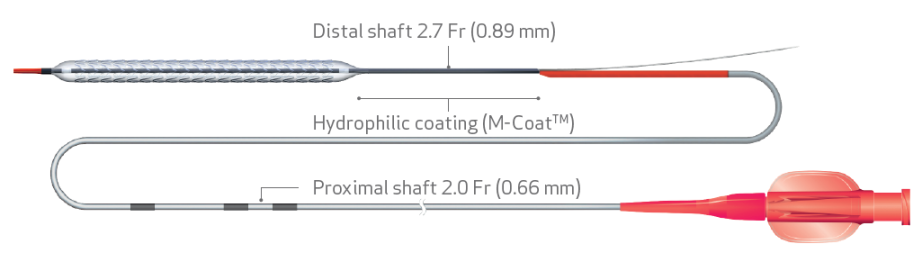
- New hydrophilic coating for enhanced deliverability10,11
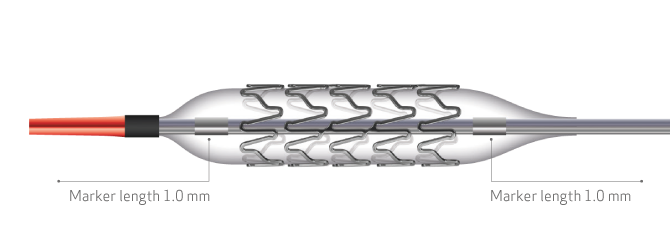
- New balloon with a nominal pressure of 11 atm for a better apposition7,12
- Increased catheter inner lumen for fast deflation time13,14
Inherited Drug and Polymer
Inherited abluminal gradient bioresorbable coating to maintain polymer integrity even when overexpanded, allowing for an optimized drug dose of sirolimus (3.9 μg/mm of stent)4,5,15,16, simultaneous polymer resorption and drug release within 3-4 months
Ultimaster Nagomi™ is building on the Heritage of Strong Clinical Evidence of the Ultimaster™ Stent Family22
- > 50,000 patients enrolled in the Ultimaster™ clinical program, covering the most complex patient subgroups17 :
- Optimal vascular repair18
- Long term safety and efficacy in RCT mirrored in real world clinical practice19,20
- 1-month DAPT in high-bleeding risk patients21
Abbreviated Antiplatelet Therapy After Coronary Stenting in Patients with Myocardial Infarction at High Bleeding Risk
C. Smits et al J Am Coll Cardiol. 2022
Duration of antiplatelet therapy after complex percutaneous coronary intervention in patients at high bleeding risk : a MASTER DAPT trial sub-analysis.
Valgimigli et al. Eur Heart J. 2022
Stent specifications
| Stent design | Open cell |
| Stent material | Cobalt Chromium L605 |
| Stent thickness | 80 μm |
| Drug | Sirolimus |
| Drug dose | 3.9 μg/mm stent length |
| Polymer | Poly (DL-lactide-co-caprolactone) |
| Drug coating | Abluminal & gradient |
| Polymer degradation time and drug release | 3-4 months |
Delivery system specifications
| Guidewire compatibility | 0.014’’ (0.36 mm) |
| Nominal pressure | 11atm |
| Rated burst pressure | 16 atm |
| Entry profile | 0.018’’ (0.45 mm) |
| Coating | Hydrophilic – Distal shaft |
| Minimum guide catheter | Minimum guide catheter 5 Fr for Ø 2.00 – Ø 4.00 mm (0.056’’/1.42 mm) 6 Fr for Ø 4.50 mm (0.071’’/1.80 mm) Usable length 144 cm |