Are you interested in short DAPT? Learn more…
What
The MASTER DAPT trial is and how it applies to short DAPT.
Why
The MASTER DAPT trial is different to other clinical studies on short DAPT?
How
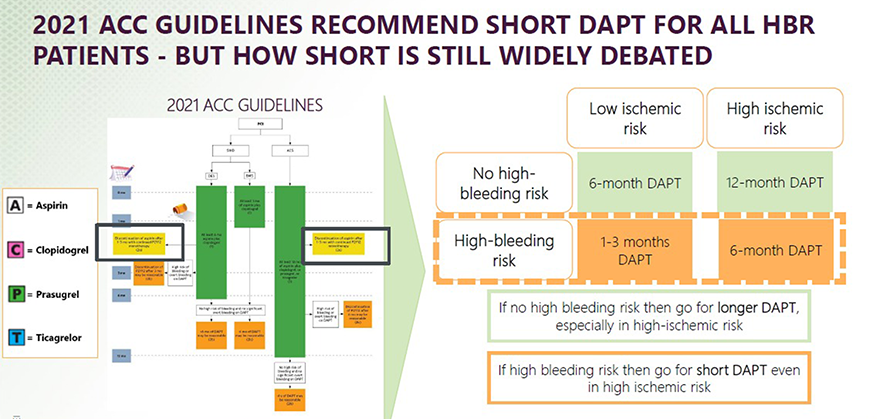
A short DAPT protocol fits with established guidelines?
Read more about the MASTER DAPT Trial
Sub-analysis: Complex PCI
Read the latest results from the MASTER DAPT Complex PCI sub-analysis.
Sub-analysis: OAC
Read the summary report for the MASTER DAPT OAC sub-analysis result.
Summary of main trial
Read the summary report of the MASTER DAPT main trial result.
Our Ultimaster™ stent, used in the MASTER DAPT trial, has three innovative features which help support a short DAPT strategy.
1. Stent Flexibility
Flexible 80μm Cocr platform with optimal conformability to reduce mechanical stress on the vessel wall.

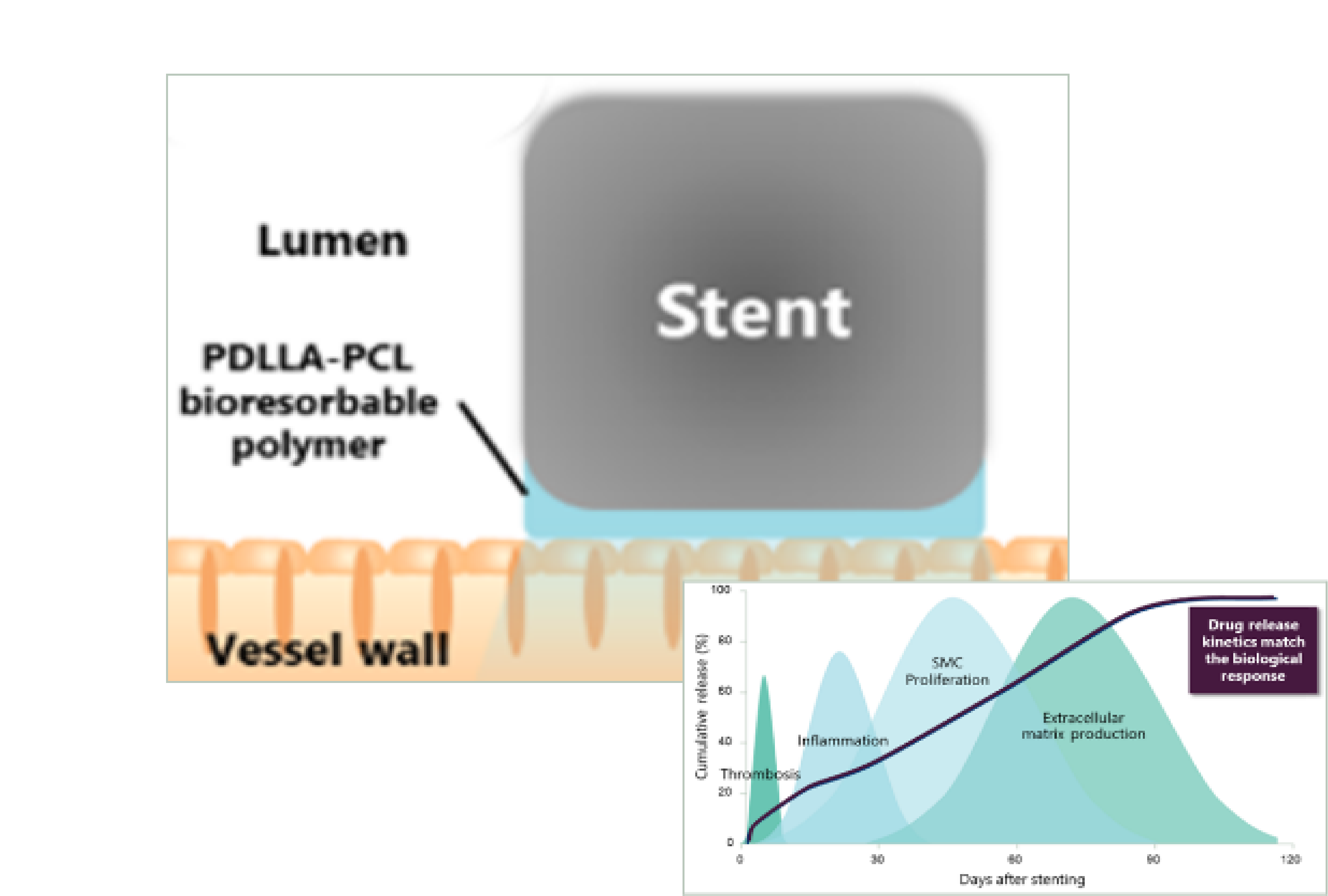
2. Gradient Coating Technology
Elastic gradient PDLLA-PCL short term polymer exposure mirrors biological response, promoting rapid vascular repair within 3-4 months.
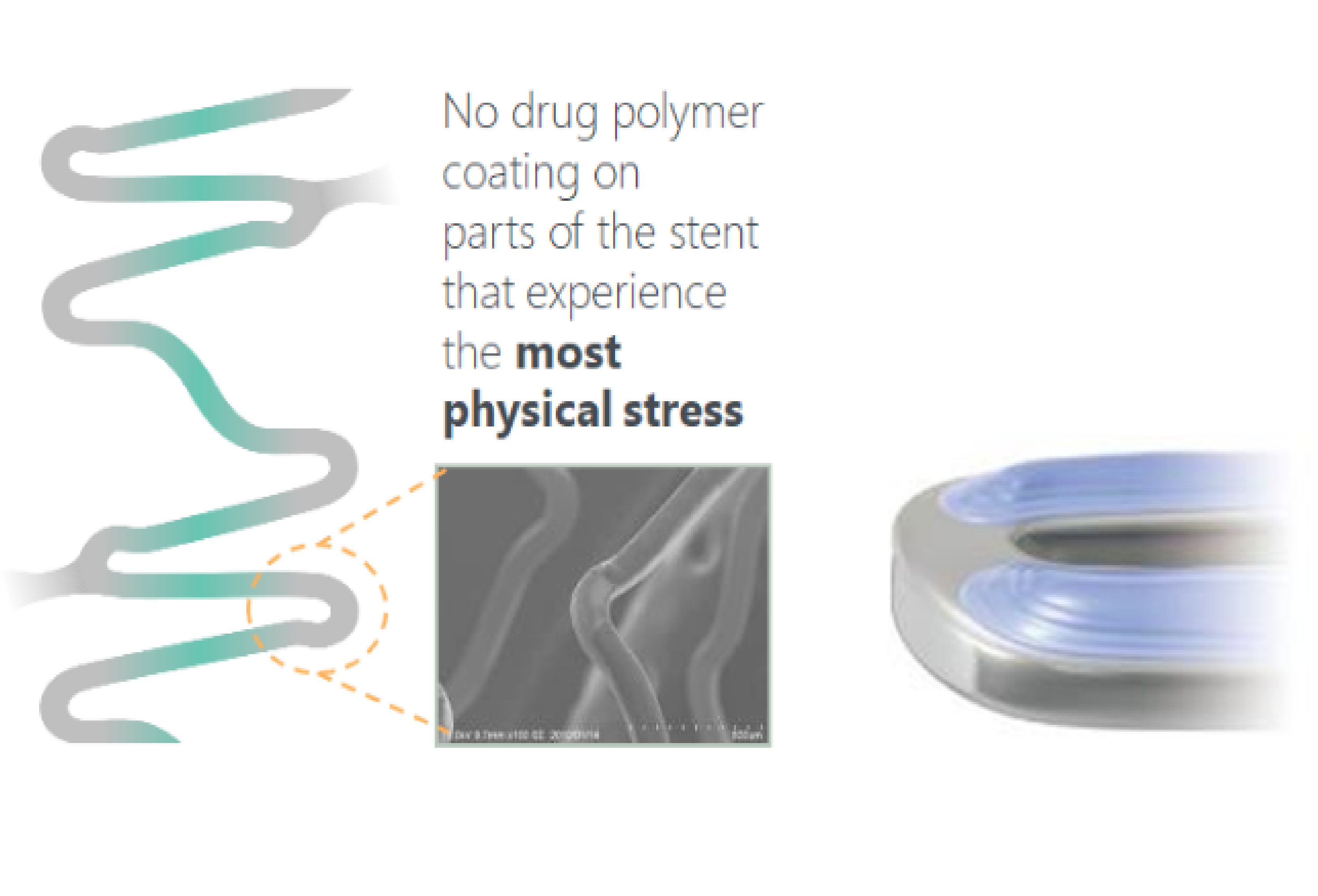
3. Abluminal Coating Technology & Drug
Silorimus: Most proven drug on stents. Drug release matches biological response targeted abluminal drug delivery. Low Nominal drug dose: 3.9μg/mm or 0.7μg/mm2
Ultimaster NagomiTM
The Ultimaster Nagomi™ is the latest generation of the Ultimaster™ DES family, bringing excellent deliverability, larger size line-up and an even greateroverexpansion capability.
Comprehensive range of clinical solution training to improve the patient experience
Terumo India offers a series of Live interactive and recorded webinars from therapy leaders and expert operators. Experience advances in procedural training, precision tools optimising your patient’s outcome and educational resources from the expert faculty with Terumo India Skill Lab through interactive & informative online .