R2V™ - Radial Solutions for Interventional Radiology
Radial to Visceral (R2V™) intervention contributes to patients' QOL. It causes fewer bleeding complications than the conventional femoral method.
Radial to Visceral (R2V™) intervention contributes to patients' QOL. It causes fewer bleeding complications than the conventional femoral method.
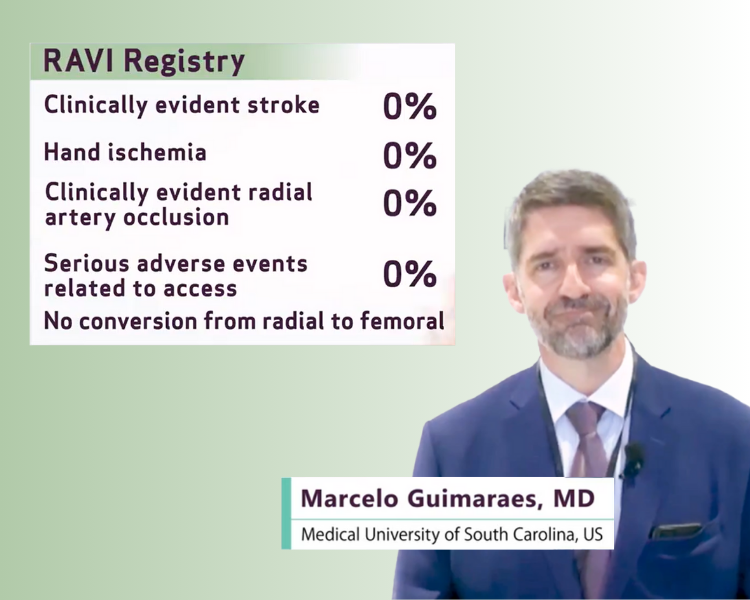
The RAVI Registry
Have you heard about the outcomes of the radial access in visceral intervention, the RAVI registry?
Learn the details from Dr. Guimaraes.
Learn the details from Dr. Guimaraes.
Have you heard about the outcomes of the radial access in visceral intervention, the RAVI registry?
Learn the details from Dr. Guimaraes.
Learn the details from Dr. Guimaraes.
Radial to Visceral
Less-invasive solution for interventional oncology – learn how we push the boundaries in introducing RADIFOCUS™ Glidecath™, the first Terumo device for radial to visceral.
Less-invasive solution for interventional oncology – learn how we push the boundaries in introducing RADIFOCUS™ Glidecath™, the first Terumo device for radial to visceral.
What is R2V™?
Radial to Visceral (R2V™) intervention brings various benefits over Transfemoral Artery Access (TFA), including reduced bleeding complications, easy access site management, improved patient satisfaction and the opportunity for same day discharge. Terumo offers various access devices and embolic agents for R2V™. 1-7
The RAVI Registry
The RAVI registry, a prospective registry on 30-day outcomes with 12 month follow up was conducted to increase the data available on radial access in visceral interventions and to show that radial access is safe and feasible for a wide range of embolization procedures.
Related Products
Related Topics
Reference
- Amin A, et al. Costs associated with access site and same-day discharge among Medicare beneficiaries undergoing percutaneous coronary intervention. JACC Cardiovasc Interv. 2017;10(4):342-51.
- Schussler JM. Effectiveness and safety of transradial artery access for cardiac catheterization. Proc (Bayl Univ Med Cent). 2011; 24(3):205-209.
- Marso S. et al. Association Between Use of Bleeding Avoidance Strategies and Risk of Periprocedural Bleeding Among Patients Undergoing Percutaneous Coronary Intervention. JAMA. 2010;303(21):2156-2164.
- Duffin DC, Muhlestein JB, Allisson SB, et al. Femoral arterial puncture management after percutaneous coronary procedures: a comparison of clinical outcomes and patient satisfaction between manual compression and two different vascular closure devices. J Invasive Cardiol. 2001;13(5):354-362.
- Valgimigli M, et al. MATRIX Trial. Radial versus femoral access in patients with acute coronary syndromes undergoing invasive management: a randomized multicenter trial. Lancet. 2015;385:2465-76.
- Rathore S. Impact of Length and Hydrophilic Coating of the Introducer Sheath on Radial Artery Spasm During Transradial Coronary Intervention A Randomized Study. JACC Cardiovasc Interv. 2010;3(5):475-83.
- https://www.cancer.gov/publications/dictionaries/cancer-terms/def/embolization