Prospective, Multi-Center, Registry Study of Radial Access Embolization Procedures: 30 Day Outcomes (RAVI Registry- NCT04272216)¹
Overview
A prospective registry on 30-day outcomes with 12 month follow up was conducted to increase the data available on radial access in visceral interventions (RAVI) and to show that radial access is safe and feasible for a wide range of embolization procedures.
Study Design
- Non-randomized study, conducted by Interventional Radiologists, sponsored by Terumo Interventional Systems.
- Left radial access used for embolotherapy procedures to treat uterine fibroids, benign prostatic hyperplasia, liver tumors, and other hypervascular tumors.
- Eligibility criteria included 18 yrs. of age, able to provide informed consent and willing to participate in a 30 day and 12 month clinical follow-up.
- Subject eligibility for radial access (RA) was assessed according to the Barbeau test (positive)2, pre-procedure ultrasound to check patency and whether the radial artery had > 1.6 mm in the antero-posterior diameter.
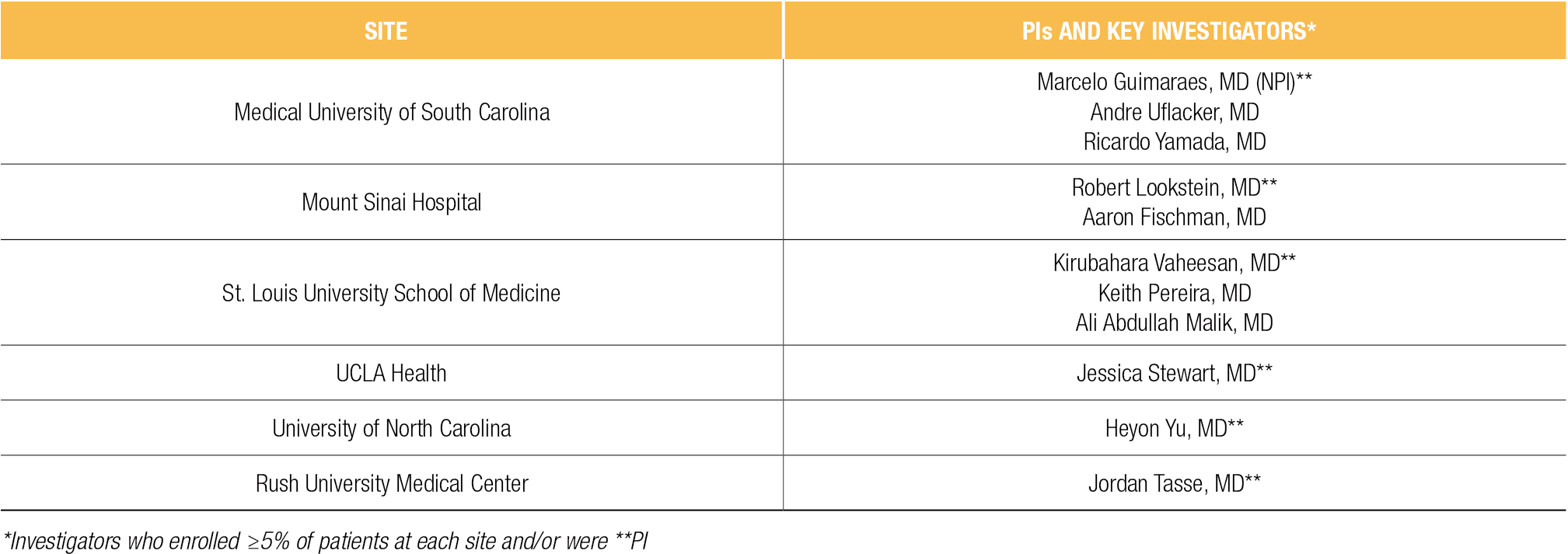
- Subjects were enrolled at six participating institutions:
Primary Endpoints
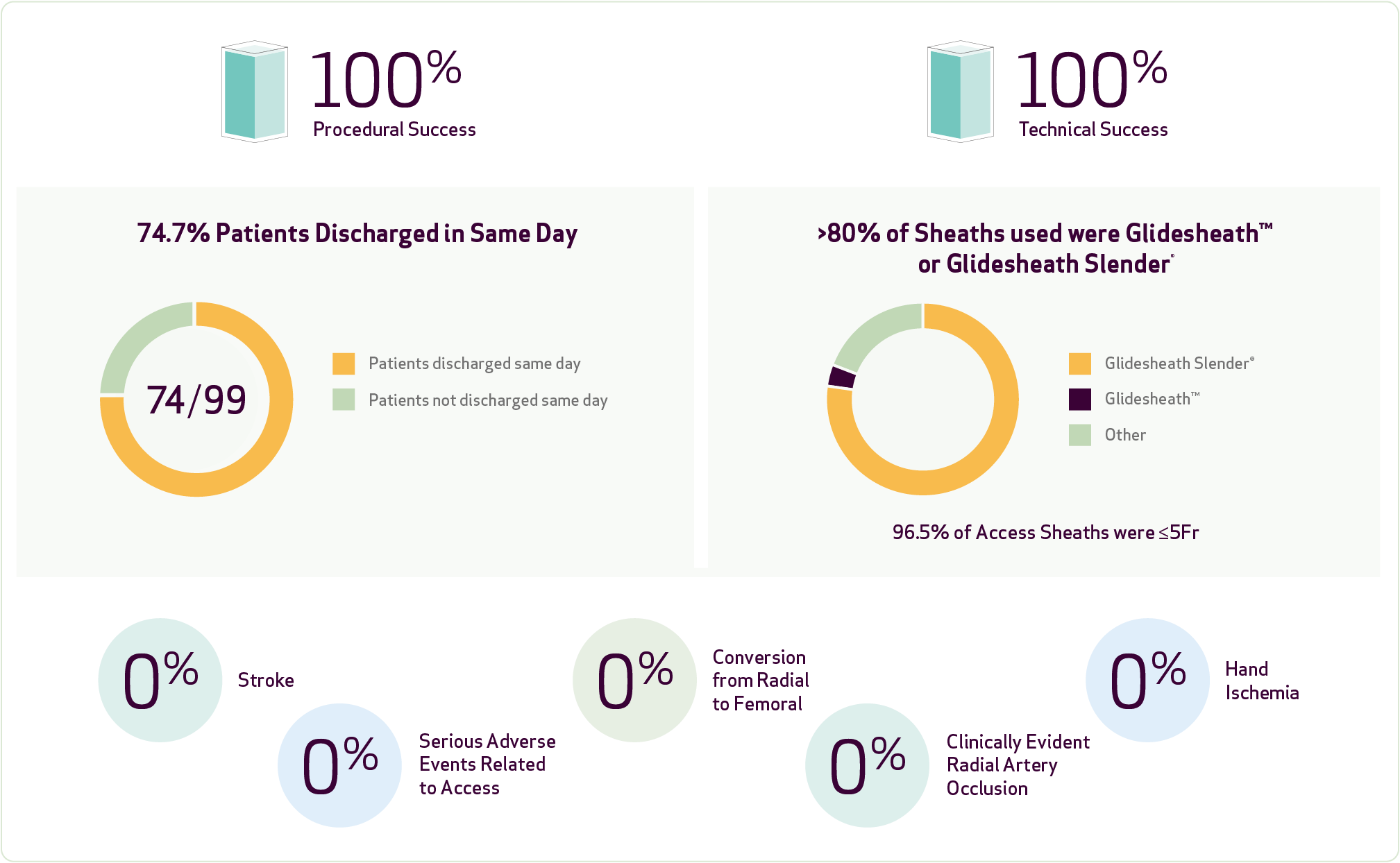
- Primary safety evaluation: Freedom from major adverse events (stroke, myocardial infarction, or death) and radial access complications (radial artery occlusion, hand ischemia, arteriovenous fistula, pseudoaneurysm), or any complication requiring surgical and/or endovascular intervention within 30 days post-procedure.
- Primary efficacy evaluation: Procedural success was completion of the intended procedure using RA without femoral bailout.
- Technical success was delivery of HydroPearl™ to the target vessel and complete embolization.
Secondary Endpoints
Radial access complications at any time, QoL, post embolization syndrome;
procedural endpoints; access endpoints; closure endpoints.
Results
- 99 patients treated between February 2020 and January 2022.
- Procedure breakdown: 71% UFE, 16% PAE, 7% liver tumor embolization, 6% other hypervascular tumor embolization.
Conclusion
This prospective registry confirmed the safety and efficacy of radial access at 30 days follow-up in embolization procedures. There was no stroke, hand ischemia or access-related death, and low rates of minor RA-related complications. Radial access is very safe and effective with no serious adverse events related to access device and can be a primary strategy for the modern embolization practice.
