Stents
Ultimaster™
Sirolimus Eluting Coronary Stent System
- The most studied BP-DES, with over 50,000 patients worldwide.
- The biggest real-world BP-DES registry shows favorable clinical performance.
- The BP-DES with the longest history.
Please contact your local representative for product details and catalogs.
Overview
Reliable Biodegradable polymer-DES
- The most studied BP-DES, with over 50,000 patients worldwide.
- The biggest real-world BP-DES registry shows favorable clinical performance.
- The BP-DES with the longest history.
Product Details
Long-term efficacy and safety proven by RCT in patients with complex background1,2
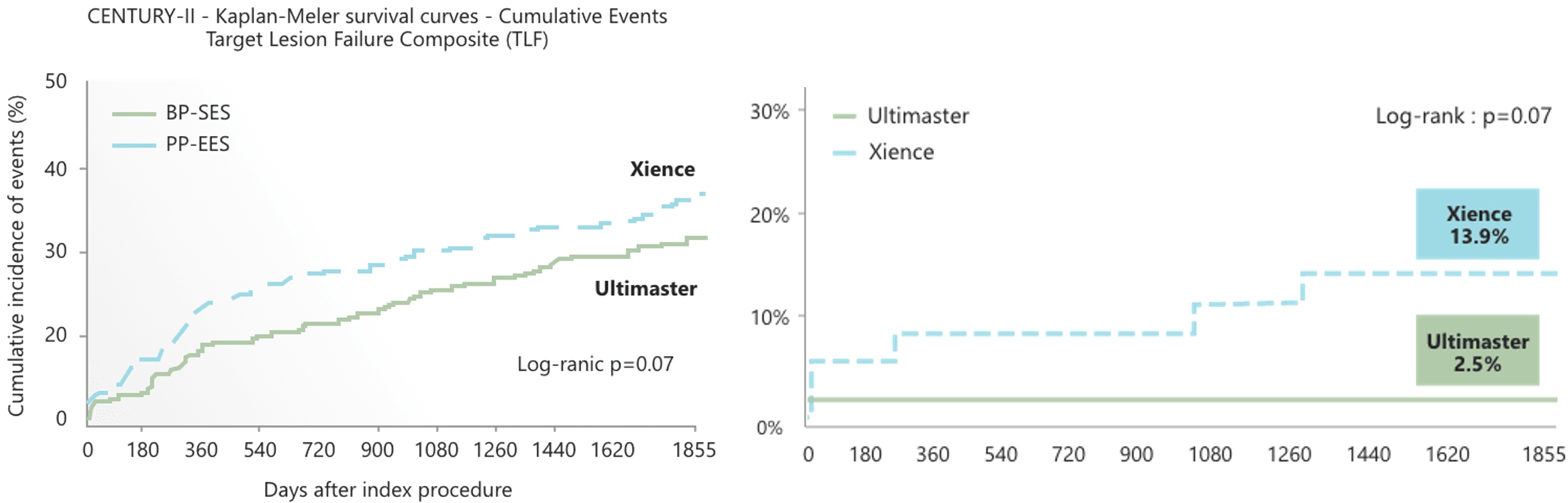
Multivessel disease patient1 & True bifurcation lesion2
CENTURY II trial is a prospective, multicenter, randomized(1:1), single-blind, controlled, non-inferiority, and two-arm trial comparing Ultimaster™ and Xience. Complex patient subset results obtained from 5-year follow up shows numerically lower fibrin area/IEL area and inflammation score with significant trend.
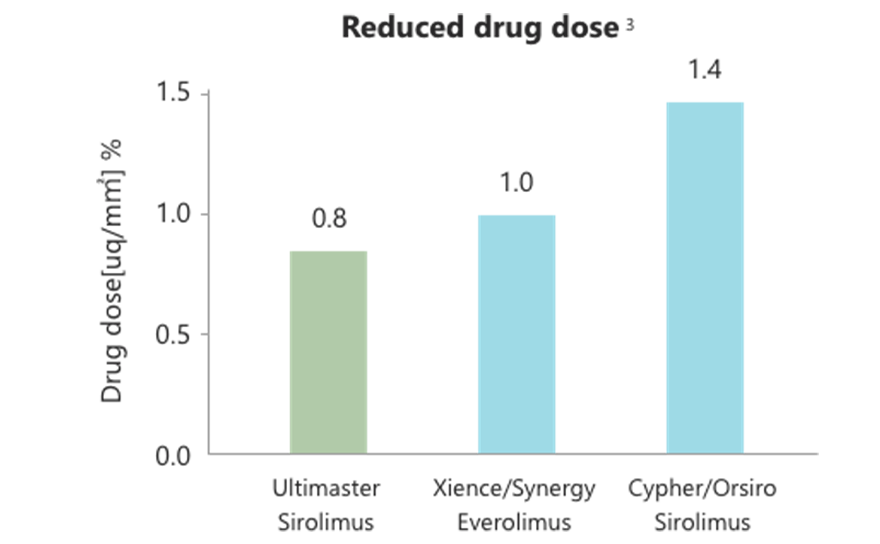
Early healing by abluminal gradient coating and reduced drug dose
- Ultimaster™ is designed to facilitate endothelialization.
- Reduced-dose Sirolimus is applied only to the abluminal side of Ultimaster™ to facilitate endothelial coverage while sufficiently maintaining neointimal hyperplasia inhibitory effect.
- Drug dose reduction minimizes inflammation caused by polymer absorption.
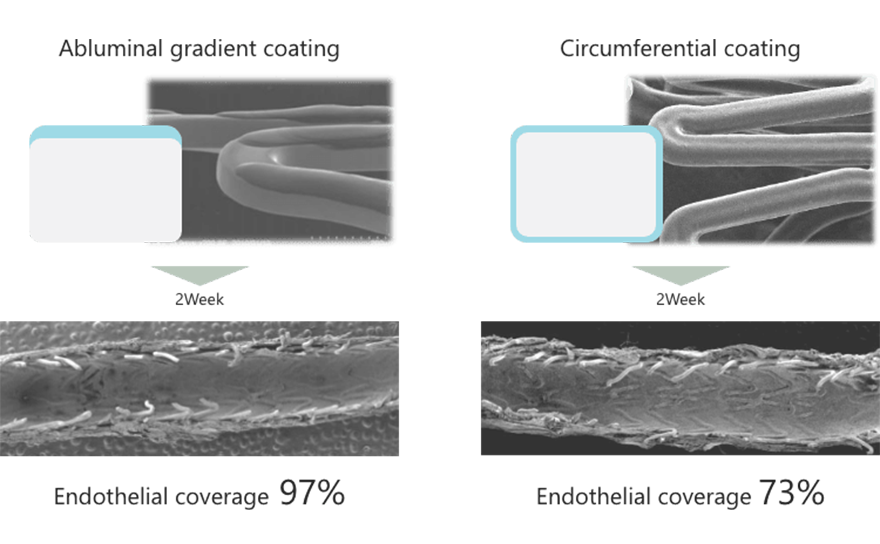
With an animal study comparing abluminal gradient coating and conventional circumferential coating, abluminal gradient coating proved useful. In this study, drug, polymer and stent platform were unified. Coating method was the only difference. Compared with circumferential coating, endothelial coverage was 32% larger for abluminal gradient coating.3
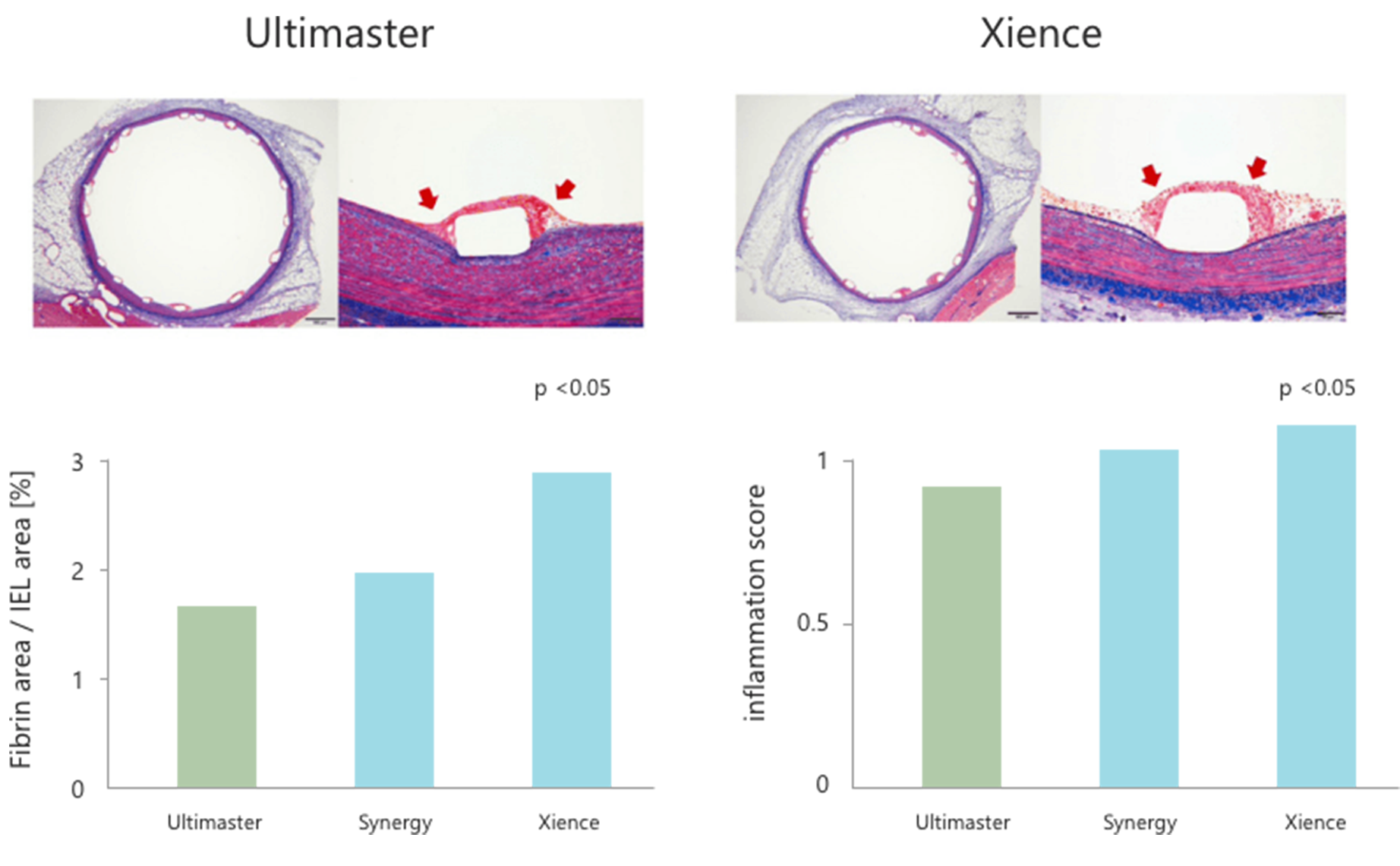
In an animal study comparing Ultimaster™ and Xience/Synergy, Ultimaster™ showed early healing.
Abluminal gradient coating reduces drug/polymer dose and facilitates endothelialization, which were proved by the fibrin area/IEL area and the inflammation score, both of which were lower for Ultimaster™ than those for other biodegradable polymer DES and durable polymer DES.4
In the DISCOVERY 1TO3 trial, Ultimaster™ showed favorable endothelial coverage even 1 month after implantation.5
Abluminal gradient coating reduces drug/polymer dose and facilitates endothelialization, which were proved by the fibrin area/IEL area and the inflammation score, both of which were lower for Ultimaster™ than those for other biodegradable polymer DES and durable polymer DES.4
In the DISCOVERY 1TO3 trial, Ultimaster™ showed favorable endothelial coverage even 1 month after implantation.5
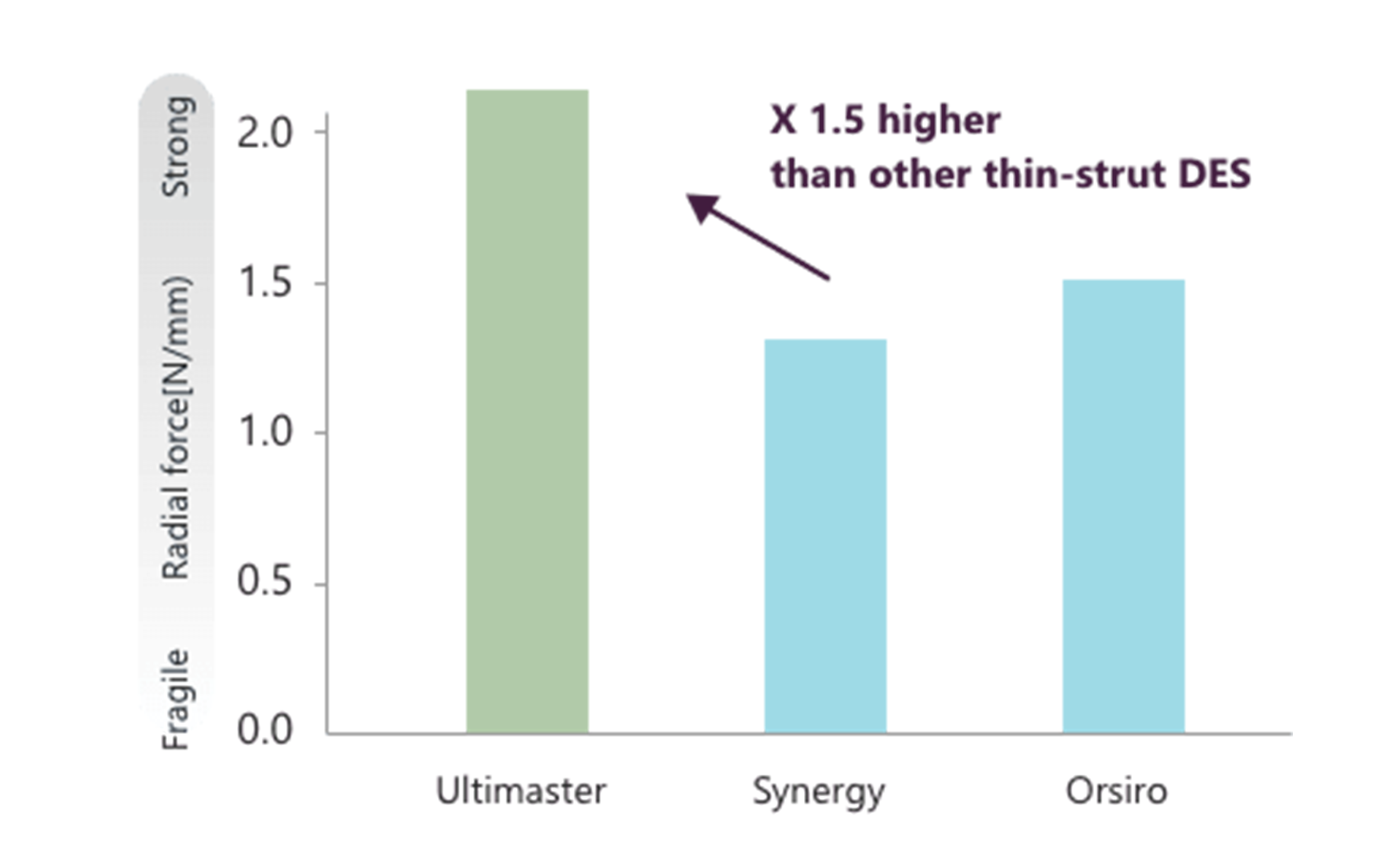
Reliable long-term patency by strong vessel support and fracture resistance
Thinner strut, but higher radial force than recent thin-strut DES.6
Stent under-expansion is one of the risk factors for restenosis and stent thrombosis. Ultimaster™ made of CoCr provides a radial force 1.5 times higher than other recent thin-strut DES.
Stent under-expansion is one of the risk factors for restenosis and stent thrombosis. Ultimaster™ made of CoCr provides a radial force 1.5 times higher than other recent thin-strut DES.
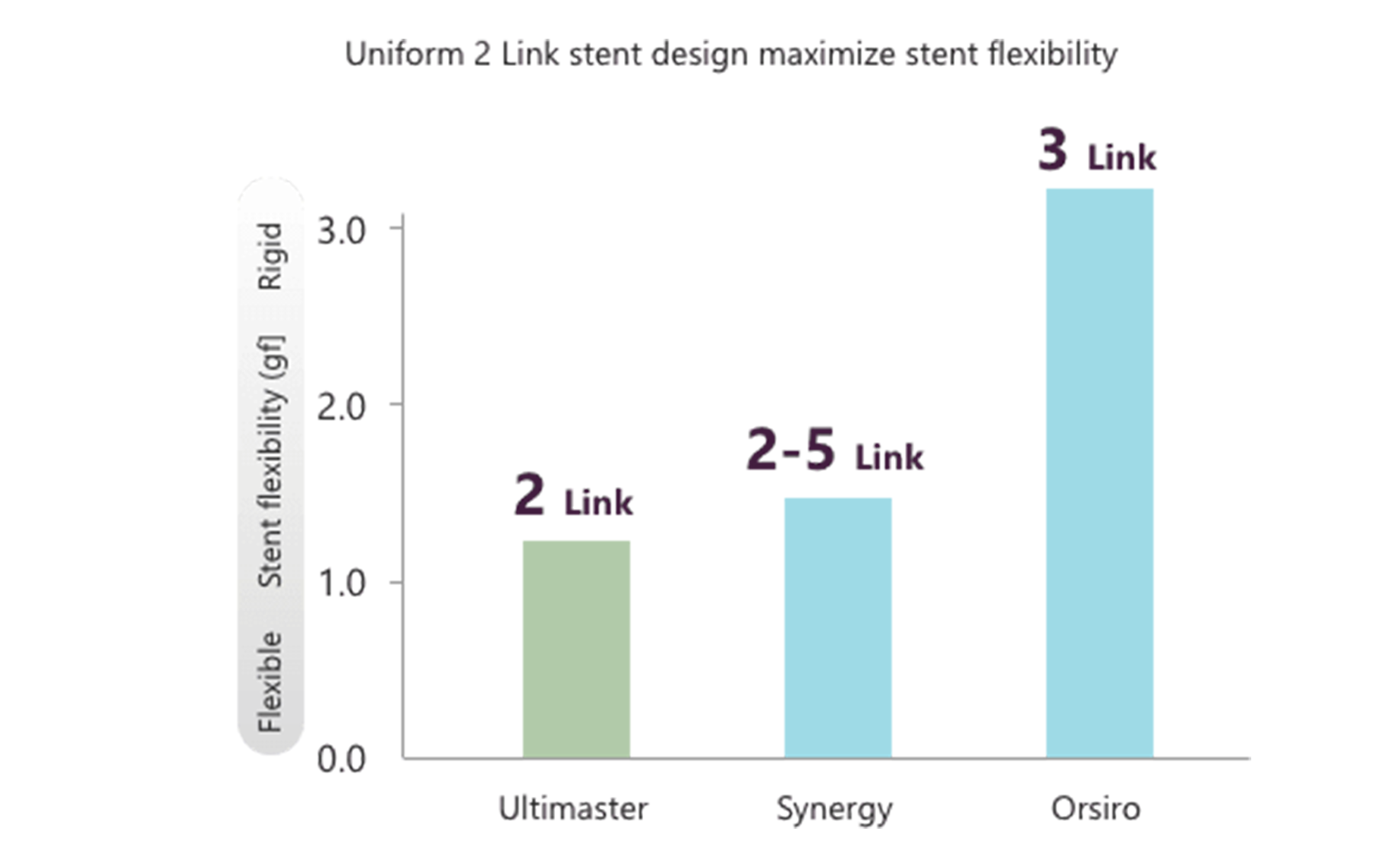
Flexible platform designed for providing fracture resistance and maintaining natural vessel geometry7
Maintaining natural vessel geometry and providing fracture resistance are essential for a stent to reduce the incidence of stent edge restenosis and maintain stent patency for a long time.
Ultimaster™'s design maximizes its flexibility to decrease fracture risks and maintain natural vessel geometry after implantation.
Ultimaster™'s design maximizes its flexibility to decrease fracture risks and maintain natural vessel geometry after implantation.
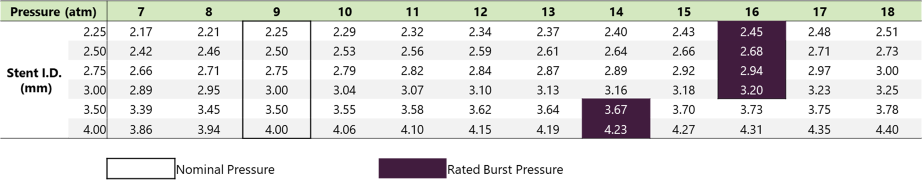
Item Specifications
Country Select
Please contact your regional representatives for more information on the products.Contact Us
| Formulation No./ Model No. | Stent diameter (mm) | Stent length (mm) | Usable catheter length (cm) | Distal shaft diameter (Fr.) | Distal shaft diameter (mm) | Proximal shaft diameter (Fr.) | Proximal shaft diameter (mm) | Marker | Nominal pressure (kPa) |
|---|---|---|---|---|---|---|---|---|---|
| DE-RD4038KSM | 4.00 | 38 | 144 | 2.6 | 0.88 | 2.0 | 0.67 | Double | 912 |
| DE-RD4038KSM | 4.00 | 38 | 144 | 2.6 | 0.88 | 2.0 | 0.67 | Double | 912 |
| DE-RD4038KSM | 4.00 | 38 | 144 | 2.6 | 0.88 | 2.0 | 0.67 | Double | 912 |
| DE-RD4038KSM | 4.00 | 38 | 144 | 2.6 | 0.88 | 2.0 | 0.67 | Double | 912 |
| DE-RD4038KSM | 4.00 | 38 | 144 | 2.6 | 0.88 | 2.0 | 0.67 | Double | 912 |
| DE-RD4038KSM | 4.00 | 38 | 144 | 2.6 | 0.88 | 2.0 | 0.67 | Double | 912 |
| DE-RD4038KSM | 4.00 | 38 | 144 | 2.6 | 0.88 | 2.0 | 0.67 | Double | 912 |
| DE-RD4038KSM | 4.00 | 38 | 144 | 2.6 | 0.88 | 2.0 | 0.67 | Double | 912 |