Vessel Occlusion using Hydrogel-Coated versus Nonhydrogel Embolization Coils in Peripheral Arterial Applications: A Prospective, Multicenter, Randomized Trial¹
Overview
To evaluate the safety and effectiveness of hydrogel-coated coils for vessel occlusion in the body trunk.
Study Design
- A Prospective, Multicenter, Randomized Trial.
- A total of 77 patients with various peripheral vascular lesions, treatable by embolization with coils, were randomized (hydrogel group, n=38; nonhydrogel group, n=39).
- In the hydrogel group, embolization of the target vessel was conducted using 0.018 inch hydrogel-coated coils (AZUR 18; Terumo Medical Corporation, Tokyo, Japan) with or without bare platinum coils.
- The nonhydrogel group received both bare platinum coils and fibered coils without the use of hydrogel-coated coils.
Result
Serious adverse events: 0
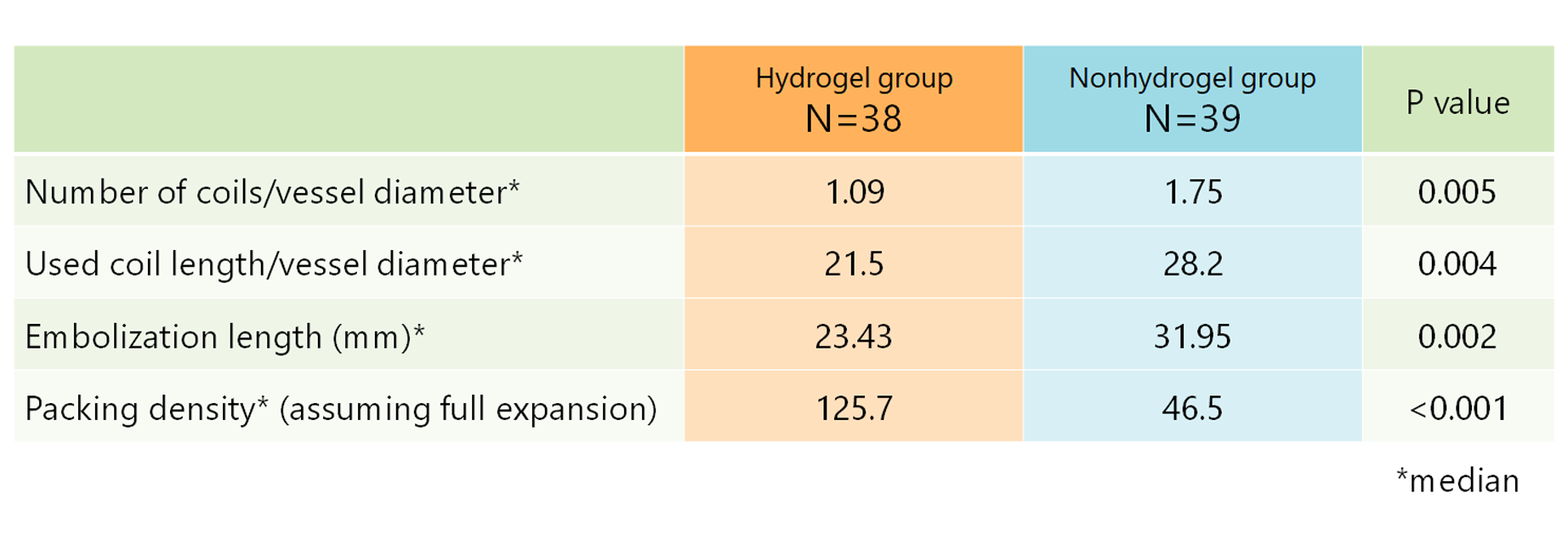
Number of coils/vessel diameter, used coil length/vessel diameter and embolization length were significant small, and packing density assuming full expansion was significantly high in hydrogel group compared with nonhydrogel group.
Conclusion
Hydrogel-coated coils can be safely used for peripheral vascular coil embolization, and hydrogel-coated and conventional coils in combination allow for a shorter embolization segment and shorter coil length.



