The COLOR(Complex Large-Bore Radial Percutaneous Coronary Intervention) trial
Overview
Randomized Comparison Between Radial and Femoral Large-Bore Access for Complex Percutaneous Coronary Intervention (Complex Large Bore Radial PCI) The COLOR trial is
- A multicenter prospective randomized trial comparing TRA1 (7Fr) vs. TFA2 (7Fr)
- Randomized 388 patients with complex PCI3 either to TRA1 (7Fr) or TFA2 (7Fr) PCI
1.TRA: Transradial access
2.TFA: Transfemoral access
3.PCI: Percutaneous coronary intervention
Study Design
| Study Type | Randomized controlled study |
|---|---|
| Actual Enrollment | 388 participants |
| Allocation | Randomized |
| Intervention Model | Parallel Assignment |
| Official Title | Complex Large-bore Radial PCI Trial Randomized Trial Reducing Access Site Complications With Slender Technology for Complex PCI*3 |
| Actual Study Start Date: | March, 2019 |
| Actual Primary Completion Date | April, 2020 |
| Actual Study Completion Date | May, 2020 |
(*3) PCI:percutaneous coronary intervention
Primary Endpoint
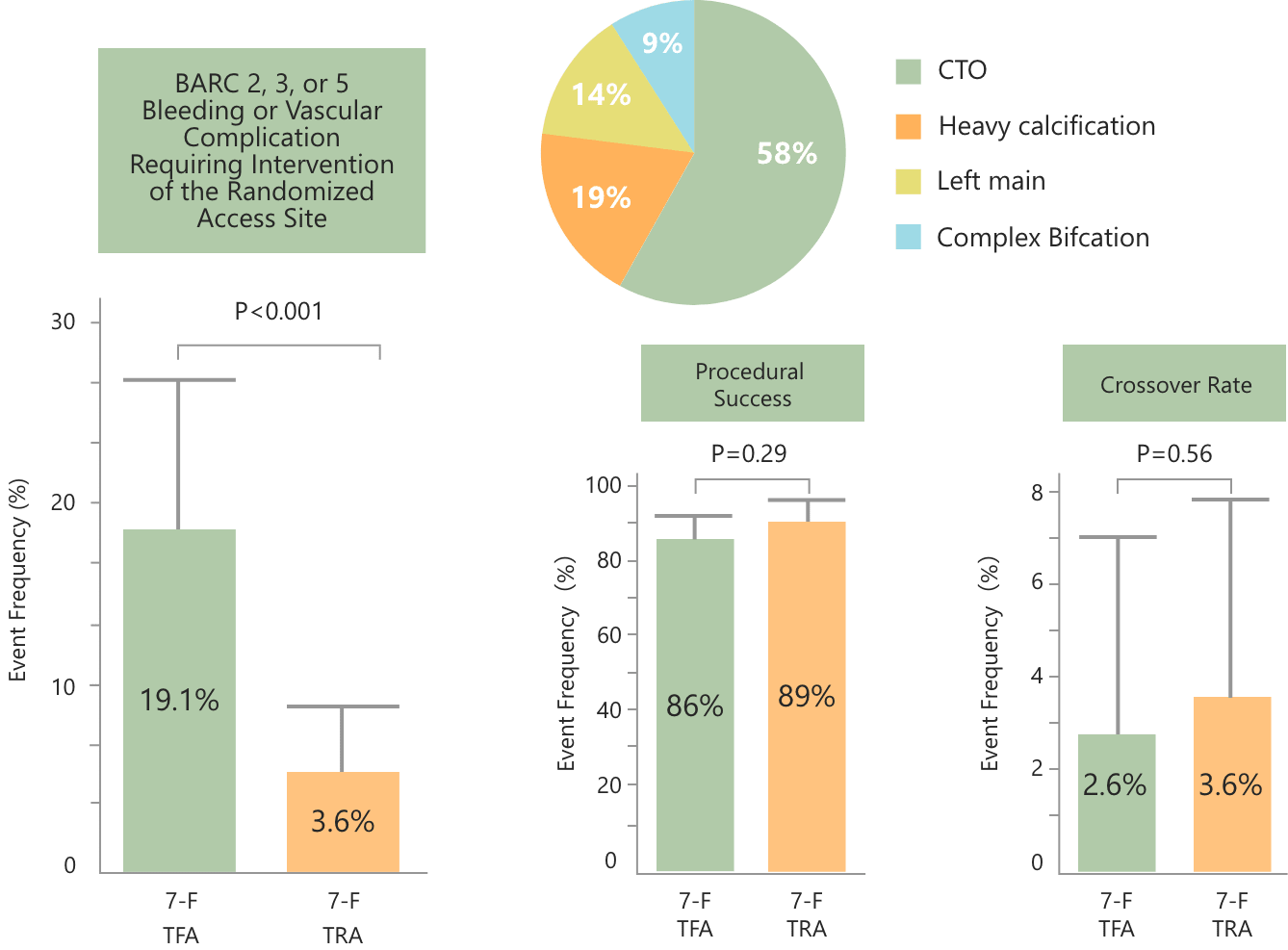
Access site–related clinically significant bleeding or vascular complications requiring intervention at discharge.
Secondary Endpoint
Procedural success (One of the secondary endpoints)
Result
The enhanced safety and comparable efficacy of 7Fr Glidesheath Slender™ (GSS) TRA over 7Fr TFA
Significant reduction of BARC 2, 3, or 5 Bleeding or Vascular Complication Requiring Intervention of the Randomized Access Site ---- 7Fr GSS TRA (3.6%) vs. 7Fr TFA (19.1%)
Comparable procedural success regardless of the selection of access site ---- 7Fr GSS TRA (86%) vs. 7Fr TFA (89%) No significant difference in procedure time, contrast use, and radiation between 7Fr GSS TRA and 7Fr TFA
The 7Fr GSS TRA is as efficacious as 7Fr TFA in successfully performing complex PCI, while enhancing its procedural safety with significant reduction of clinically relevant access-site related bleeding or vascular complications.