FAQ about Ultimaster™
Frequently Asked Questions.
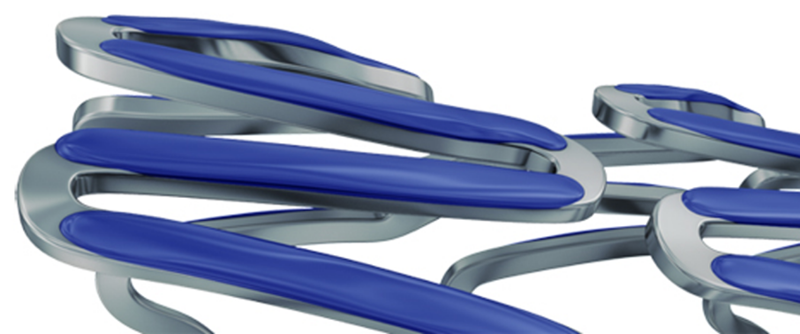
Why is Sirolimus applied to Ultimaster™/Ultimaster™ Tansei?
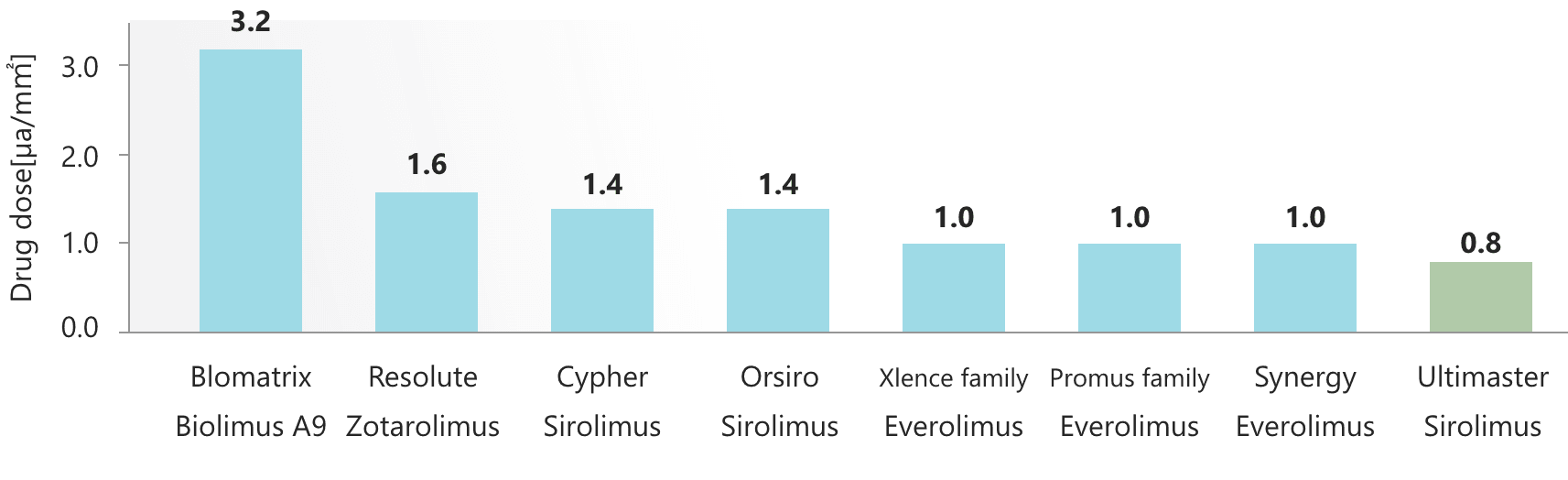
Sirolimus has a higher drug titer than Everolimus and Biolimus A9.1 Compared with competitors, Ultimaster™ (sirolimus-eluting stent) can reduce the drug dose while maintaining a sufficiently high drug titer.2 Drug dose reduction leads to polymer dose reduction, minimizing inflammation caused by polymer degradation.
Click to see more information on Ultimaster™
1. Kozuma K: Basic components of drug-eluting stent. J Jpn Coron ASSOC 2005; 11:121 125
2. Instruction for use and presentation by Virmani, EuroPCR 2013
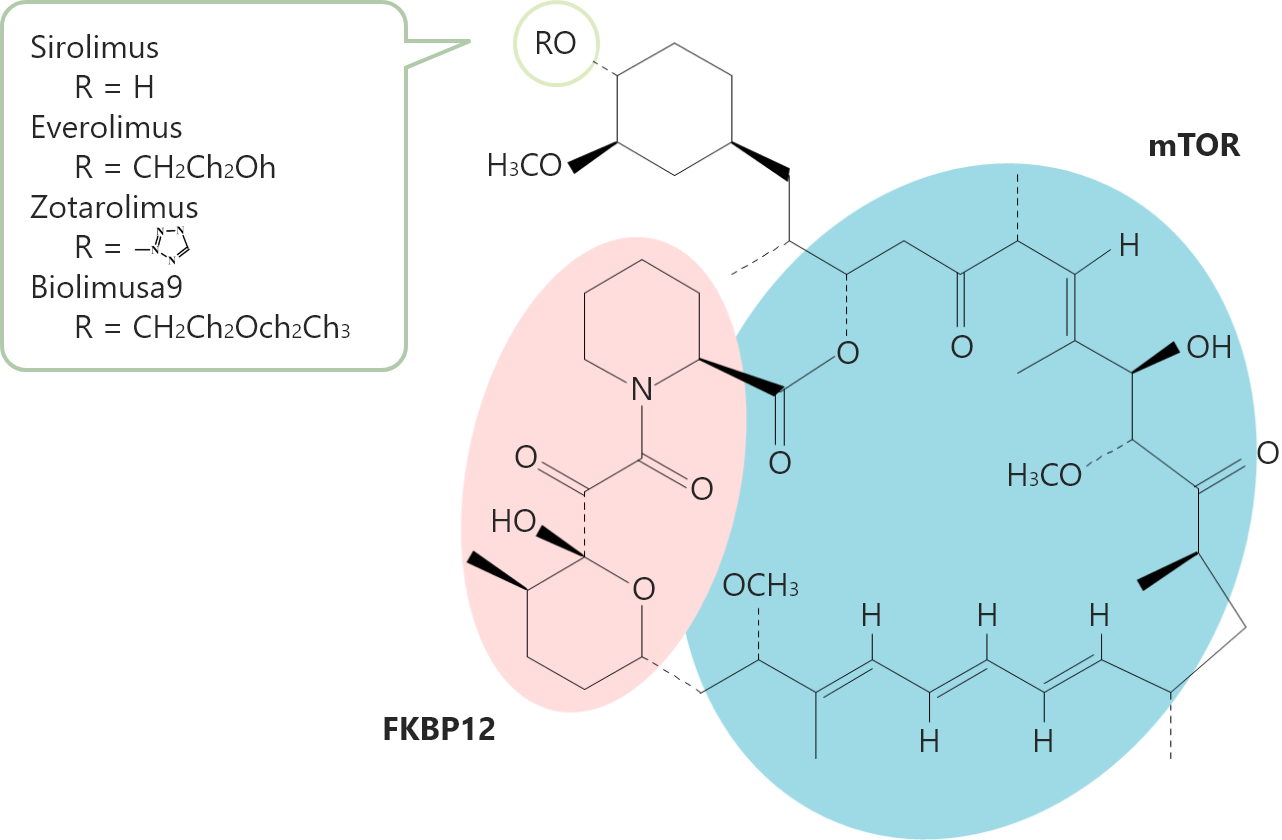
What is the difference between Sirolimus and other drugs?
With the same action mechanism, every limus-family drug originates from Sirolimus. Each limus-family drug is produced only by replacing the R radical of the macrolide. Every limus-family drug has the same mTOR and FKBP12 structures. Sirolimus has a higher drug titer than Everolimus and Biolimus A9.1 Compared with competitors, Ultimaster™ can reduce the drug dose while maintaining a sufficiently high drug titer.2
2. Instruction for use and presentation by Virmani, EuroPCR 2013
Which polymer is applied to Ultimaster™/Ultimaster™ Tansei?
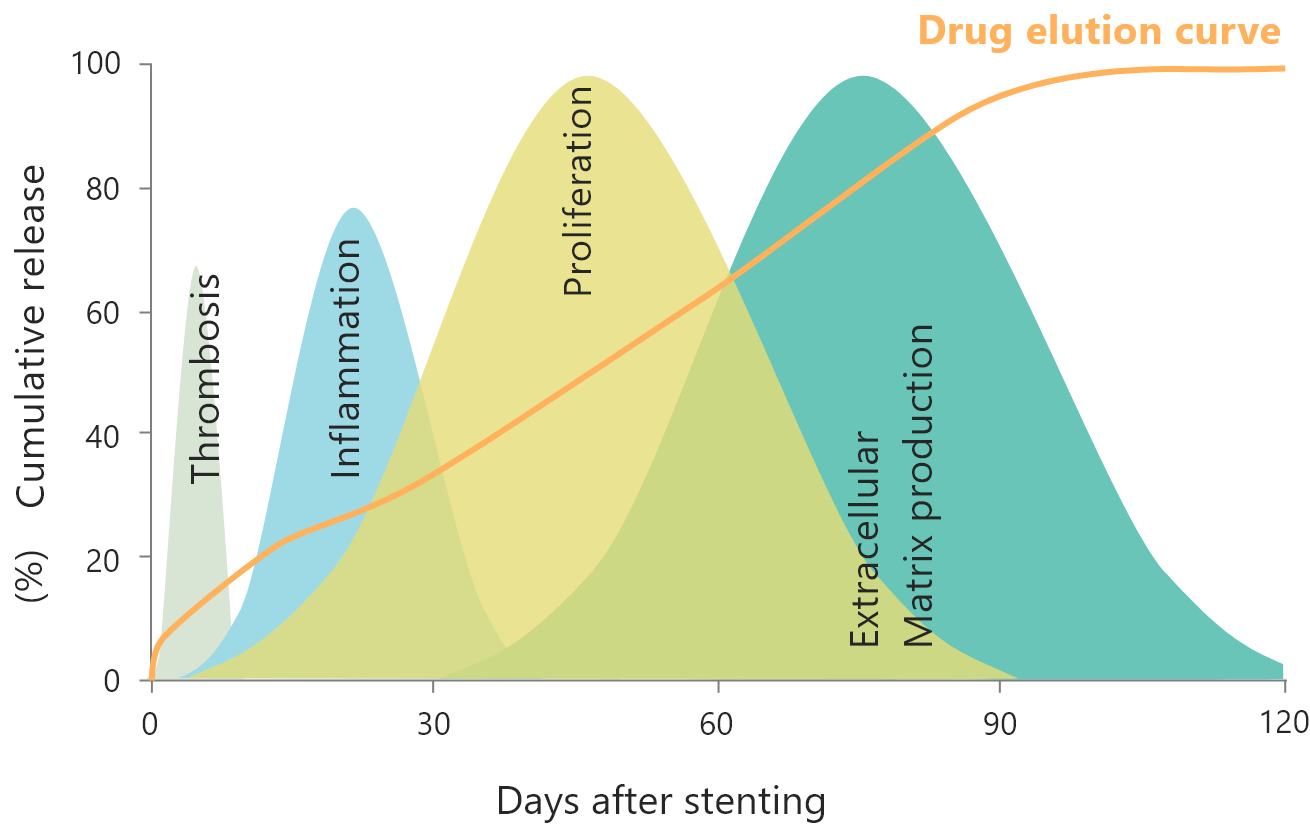
PDLLA-PCL polymer is coated on the abluminal side of Ultimaster™. Combining PDLLA and PCL gives Ultimaster™ a strong and elastic polymer to support appropriate drug release within 3-4 months after implantation. To reduce vascular inflammation and facilitate rapid vascular repair, it is essential to eliminate long-term exposure to the drug and polymer.
What is the thickness of the drug and polymer coating?
For reliable drug delivery, Terumo has applied “gradient coating technology” to the drug and polymer coating. The thickest part of the coating is 15μm in thickness.
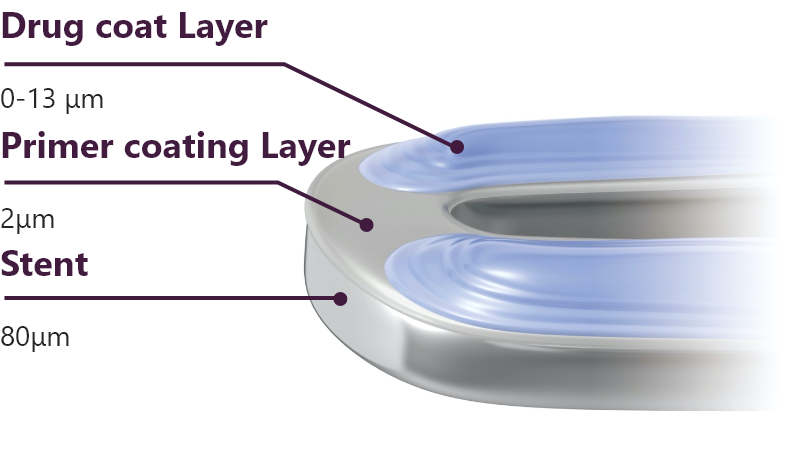
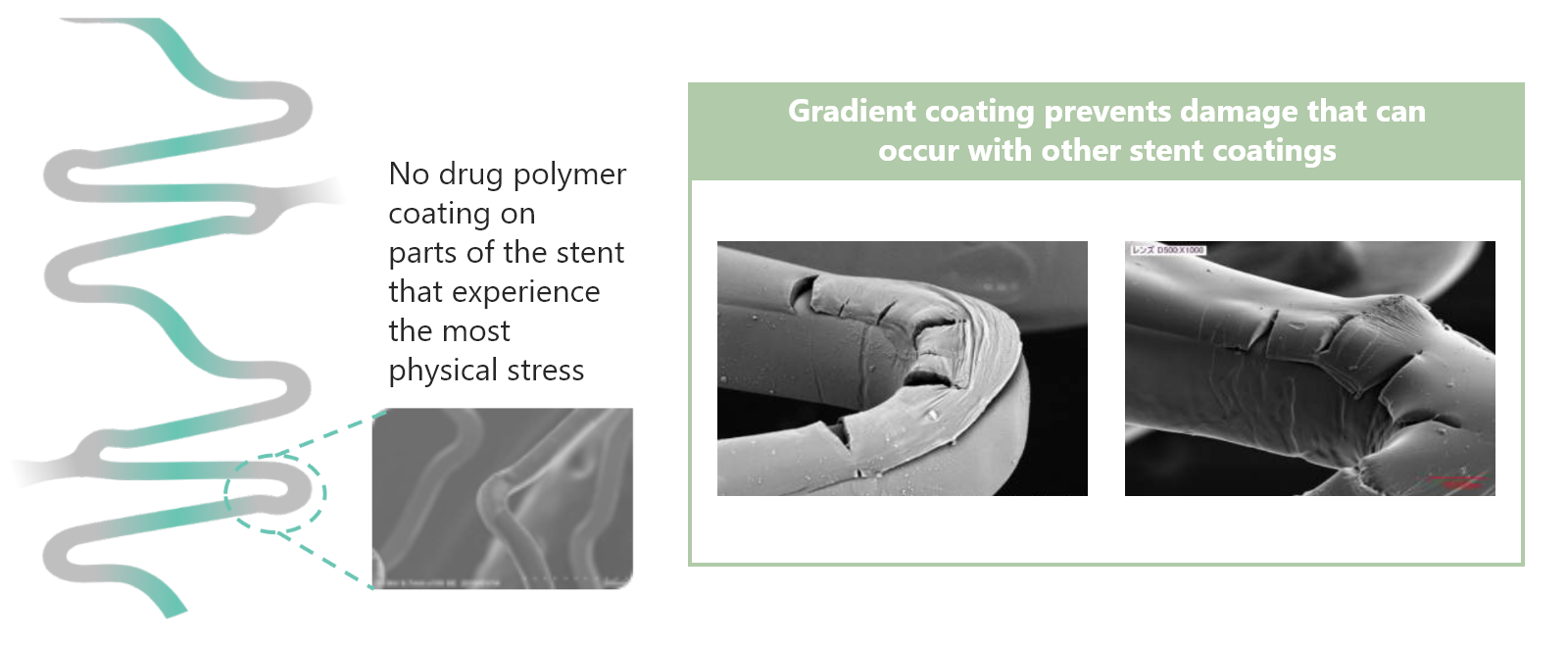
Why is “gradient abluminal coating” applied to Ultimaster™/Ultimaster™ Tansei?
Gradient abluminal coating allows reliable drug delivery leading to minimization of polymer cracking.1
Click to see more information on publication
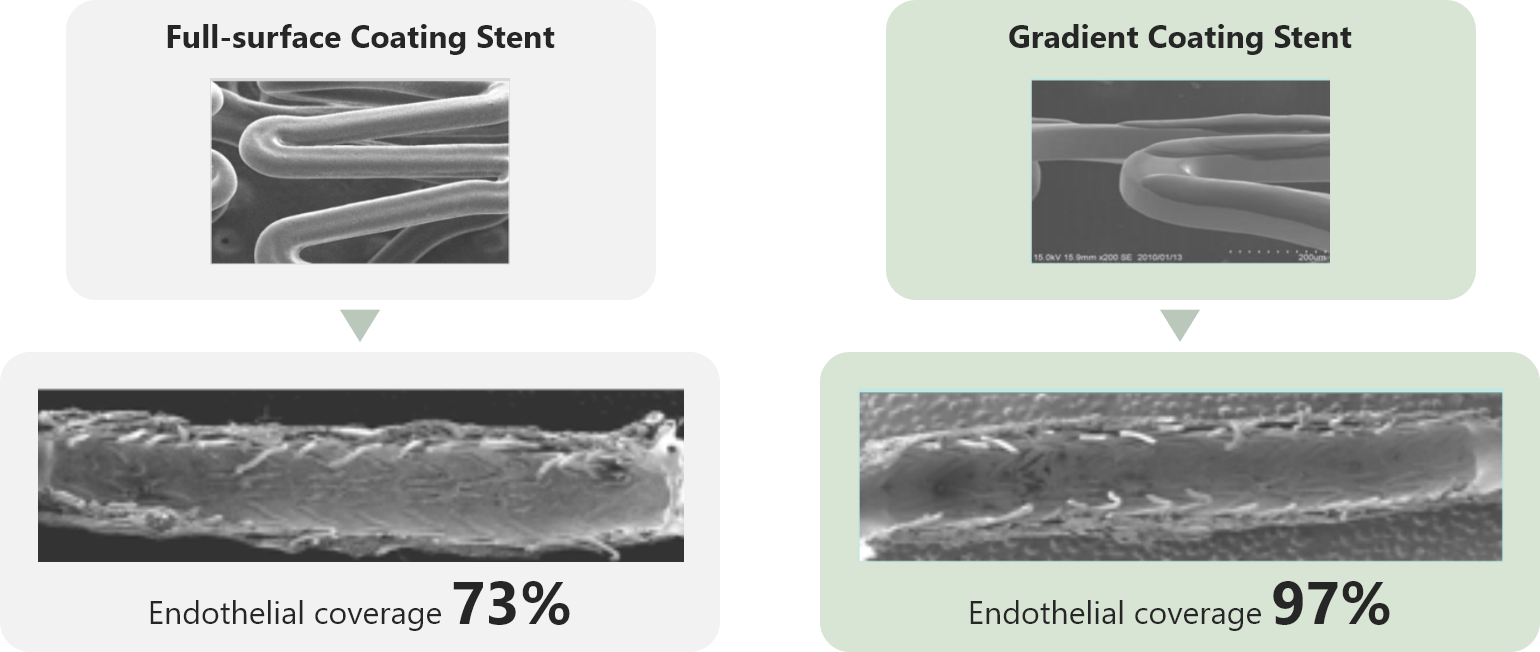
Is there any evidence supporting gradient abluminal coating?
Gradient coating reduces the risk of polymer cracking and secures reliable drug delivery. An animal study showed that gradient abluminal coating facilitates endothelialization compared with full-surface coating.1
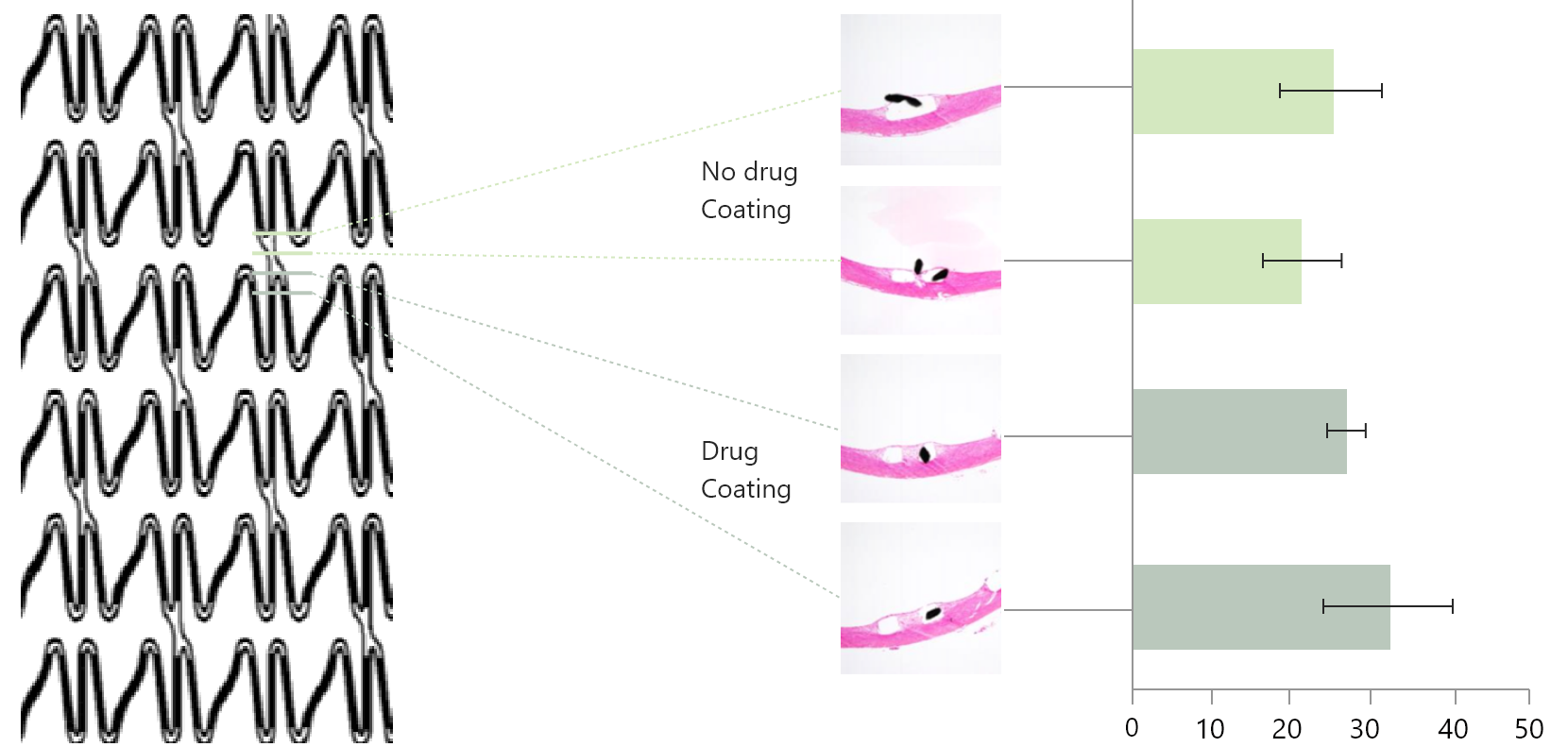
How does Ultimaster™ secure proper drug delivery to the non-drug-coated part of the stent system?
Stent metal generally covers 10-20% of the target vessel surface area. The drug diffuses and reaches the area uncovered by the metal. The 28-day results of a study using the iliac artery of rabbits showed that gradient abluminal coating allows drug delivery to the non-drug-coated part of the stent and prevents neointimal hyperplasia.1
Click to see more information on publication
How long will DAPT be continued after Ultimaster™/Ultimaster™ Tansei implantation?
Patients should be maintained on clinically adequate post-procedural antiplatelet therapy (aspirin, thienopyridine or other appropriate antiplatelet agents) according to the current guidelines. (In case of need, dual antiplatelet therapy can be discontinued earlier, but not before one month.1)
Terumo has received 1 month DAPT CE Mark approval for Ultimaster™ family DESs based on a pooled analysis regarding short DAPT. A MASTER DAPT clinical trial is ongoing which compares abbreviated and prolonged DAPTs after Ultimaster™ implantation in high bleeding risk patients.2
[Click to see more information on MASTER DAPT]
1.Terumo Ultimaster™ and Ultimaster™ Tansei instruction for use
2. Press release by Terumo Corp. in Nov. 2019
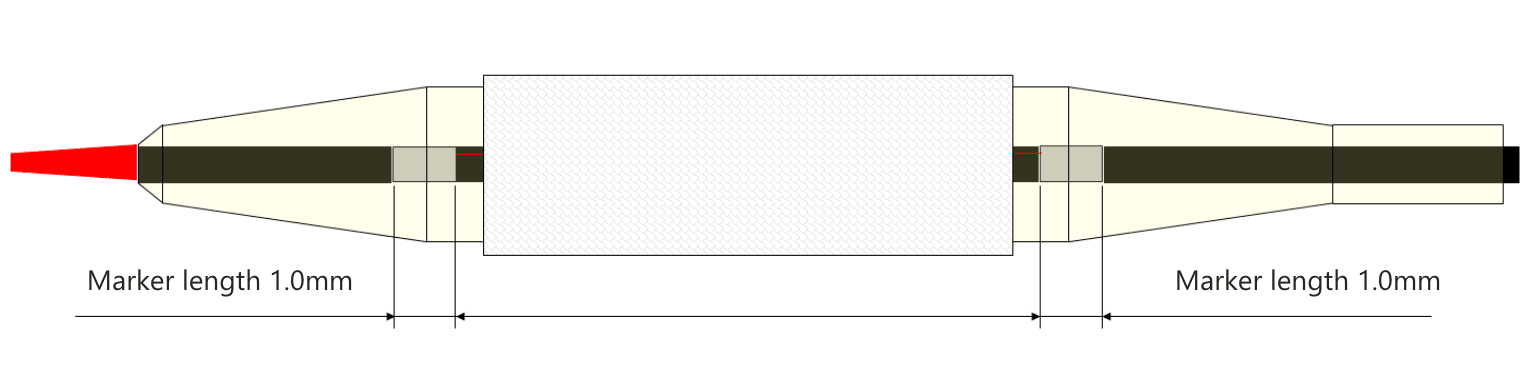
How are the marker length and the stent crimping position ?
• Marker length is designed to be 1.0mm.
• Ultimaster™ stent is crimped so that it is positioned at the center between two radiopaque markers.
How is the MRI compatibility of Ultimaster™/Ultimaster™ Tansei after implantation?
Non-clinical testing has demonstrated that the Ultimaster™ Sirolimus Eluting Coronary Stent, overlapped configuration (max. 2 stents × max. OD4mm× 38mm = 73.6mm total length) is MR conditional.
It can be scanned safely under the following conditions:
MR Conditional for
• static magnetic field of 1.5 Tesla and 3 Tesla only, with
• spatial gradient field of 36 T/m and less
• spatial gradient field product of 99 T2/m and less
• theoretically estimated maximum whole body averaged (WBA) specific absorption rate (SAR) of
< 2 W/kg at 1.5 Tesla, (related to 3.6°C temperature increase; allowable level in accordance with CEM43 concept), 73.6 x 4.0 mm, overlapped configuration
< 2 W/kg at 3 Tesla, (related to 3.6°C temperature increase; allowable level in accordance with CEM43 concept), 73.6 x 4.0 mm, overlapped configuration
for 15 minutes of continuous MR scanning.
Temperatures and SAR have been derived from computer modeling with realistic human anatomy (no cooling effects considered).
MR image quality is compromised if the area of interest is in the same area or relatively close to the position of the device. Therefore, it may be necessary to optimize MR imaging parameters for the presence of this implant.1
1.Terumo Ultimaster™ and Ultimaster™ Tansei instruction for use
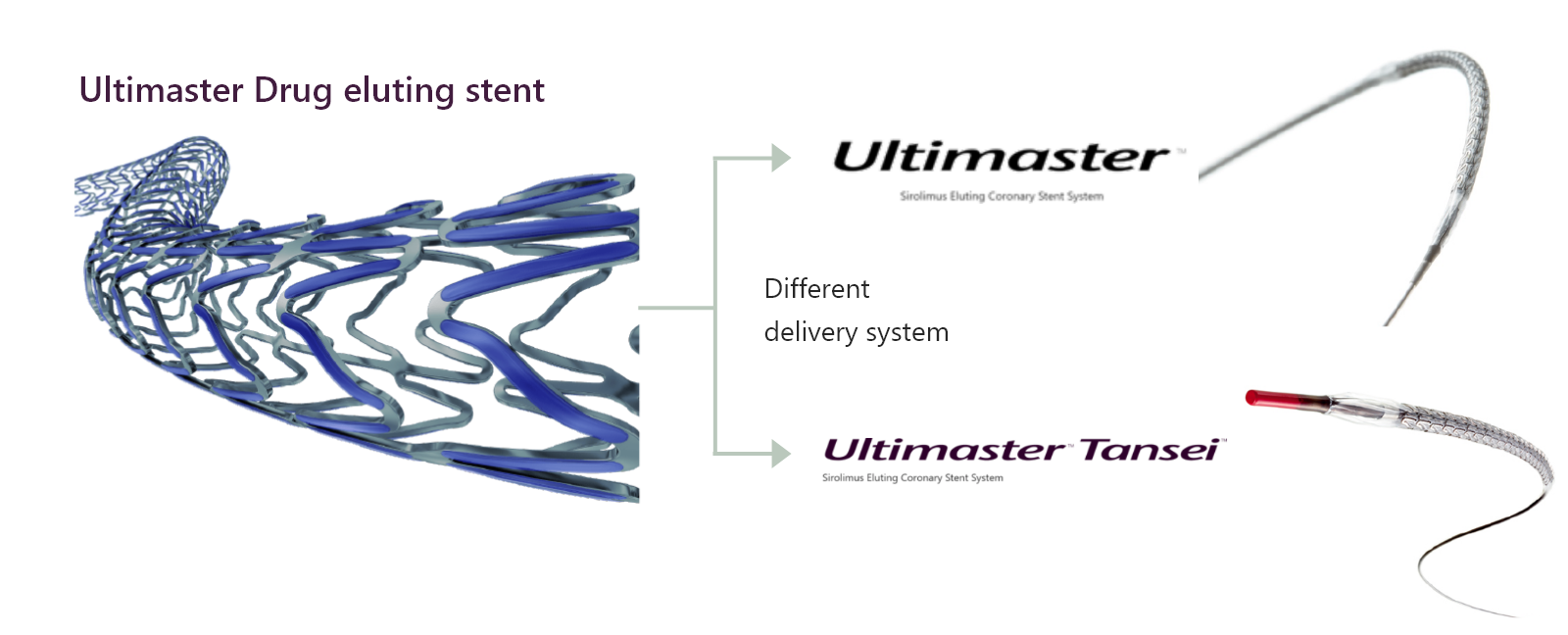
What is the difference between Ultimaster™ and Ultimaster™ Tansei?
Ultimaster™ and Ultimaster™ Tansei mount the same type of stent (the drug and polymer are the same), but use delivery systems different from each other. Ultimaster™ Tansei is designed for treatment of more complex lesions.
Which patient backgrounds are contraindications to Ultimaster™/Ultimaster™ Tansei implantation?
Contraindications1
• Patients in whom anti-platelet and/or anti-coagulant therapy is contraindicated.
• Patients with lesion(s) that prevents complete inflation of an angioplasty balloon.
• Patients with known allergy to L605 cobalt-chromium alloy.
• Patients with known allergy to nickel.
• Patients with known hypersensitivity to sirolimus or its structurally related compounds.
• Patients with known hypersensitivity to lactide polymers and caprolactone polymers.
• Patients with known hypersensitivity to contrast agent that cannot be controlled prophylactically prior to Ultimaster™ Tansei Sirolimus Eluting Coronary Stent implantation.
• Patients with extreme vessel tortuosity that may impair stent placement.
Recommendations1
• It is strongly recommended not to implant this stent in women who are pregnant.
• Effects of sirolimus during lactation have not been evaluated. Therefore it is strongly recommended to avoid breast feeding when this stent is implanted
Which metal alloy is used for Ultimaster™/Ultimaster™ Tansei ?
Ultimaster™ and Ultimaster™ Tansei are made of Cobalt Chromium L605. Terumo selected CoCr L605 to provide these devices with a thin-strut design and high flexibility for optimal crossability and conformability, and to allow them to maintain good expansion capacity while preserving a high radial force.