Products for R2V™
(Radial to Visceral)
You can complete R2V (Radial to Visceral) procedurefrom vessel access to hemostasis with Terumo’s products.
*Click to check the lineup for each procedural step.
Vessel Access
When you try to access radial artery, the quality of sheath introducer is important to avoid damage to vessel and pain for patients.
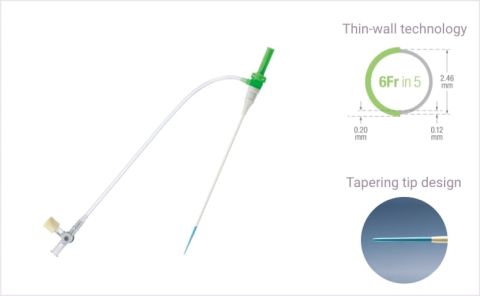 Thin-wallHydrophilicTIF-tip
Thin-wallHydrophilicTIF-tipSlender hydrophilic coated sheath for radial access
Ultra-thin wall leading to a 1 Fr reduction in outer diameter.
Designed to minimize mechanical irritation to the artery.
Results in less penetration resistance than conventional sheaths.1,2
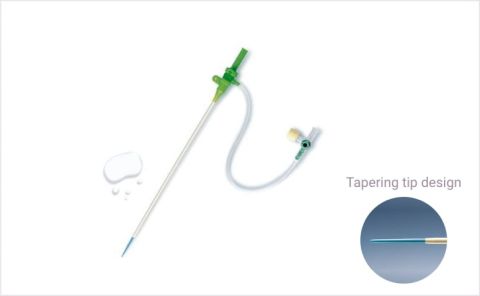 HydrophilicTIF-tip
HydrophilicTIF-tipHydrophilic coated sheath for radial access
Easy insertion and removal with proprietary Terumo Mcoat™ hydrophilic coating.
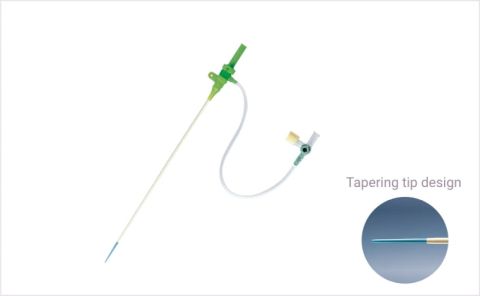 TIF-tip
TIF-tipStandard introducer sheath kit
Total Integrated Fit (TIF) tip tapering: tapering design at the tip of the sheath and dilator to facilitate smooth penetration.
Catheter Insertion
Radial artery is small and sometimes there are loops or tortuous in the vasculature. Guidewire’s smooth navigation may helps to minimize the risk of vessel trauma.
 Double braidHydrophilic
Double braidHydrophilicDedicated shape/length for R2V.
The material/constructions for good torque control and trackability.
 Nitinol coreHydrophilic
Nitinol coreHydrophilicHydrophilic coating (Mcoat™) for high lubricity with less friction.
Super elastic nitinol core provides excellent tip shape memory.
Gentry taperd tip aiming to the flexibility for easy artery navigation
Approach to Visceral
Provides excellent vessel trackability due to catheter flexibility and Terumo’s M-coat
 Nitinol coreHydrophilic
Nitinol coreHydrophilicSmall diameters and distal pre-shaped curves for super-selective access in distal and tortuous vessels
The tapered tip is designed to be highly flexible for atraumatic and controlled navigation through vessels
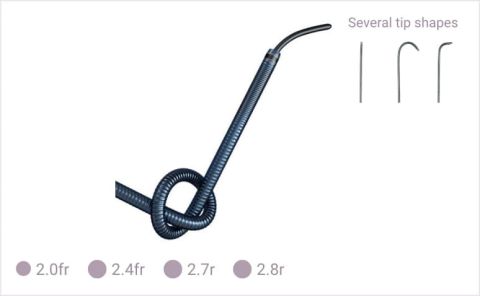 HydrophilicMicrocatheters
HydrophilicMicrocathetersTerumo's coaxial system allows fast and smooth procedures through wire integration and easy microcatheter preparation.
The tungsten spiral coil in its three layer structure protects inner lumen integrity for smooth delivery of all types of embolic materials.3
The varied pitch of the tungsten coil with Terumo's hydrophilic coating provides enhanced flexibility, thus notably increasing its distal selectivity in tortuous and narrow vessels.3
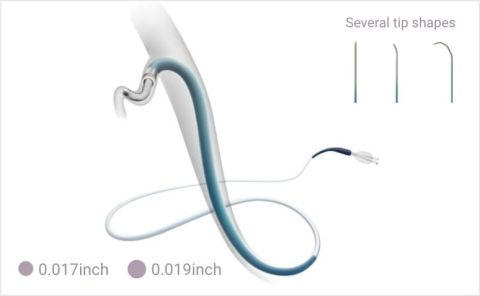 HydrophilicMicrocatheters
HydrophilicMicrocathetersTapered inner lumen
Densely-pitched asymmetric braids maintain the inner lumen for consistent embolization
Tip design to enhance flexibility
 HydrophilicMicro Balloons
HydrophilicMicro BalloonsOffers the ability to redistribute blood flow and access the microvascular circulation, leading to an increased accumulation of therapeutics into the target lesion with minimal off-target embolization.s
Excellence in lesion access technology guarantees a superb precision and accuracy in navigation through complex vessel anatomies.
Embolization
Terumo offers a broad range of embolization solutions to meet the needs of your practice.
 HydrophilicEmbolization Beads
HydrophilicEmbolization BeadsPEG (polyethylene glycol), biocompatible, compressible, non-drug-loadable and precisely calibrated embolization microspheres.
 HydrophilicEmbolization Beads
HydrophilicEmbolization BeadsPEG (polyethylene glycol) embolization microspheres that can be loaded with chemotherapeutic agents.
 HydrophilicEmbolization Coils
HydrophilicEmbolization CoilsPlatinum coil and an expandable hydrogel polymer. The hydrogel coating expands in the direction of less resistance to fill space when introduced into the bloodstream.
 HydrophilicEmbolization Coils
HydrophilicEmbolization CoilsThe first and only peripheral hydrogel coil with cross-sectional coverage and the benefits of patented hydrogel technology.
 HydrophilicEmbolization Coils
HydrophilicEmbolization CoilsA platinum coil that offers a three-dimensional shape to cover the wall of an aneurysm or the inner lumen of an artery.
Hemostasis
Dedicated compression device will help you to observe the puncture site and control the compression pressure during hemostasis.
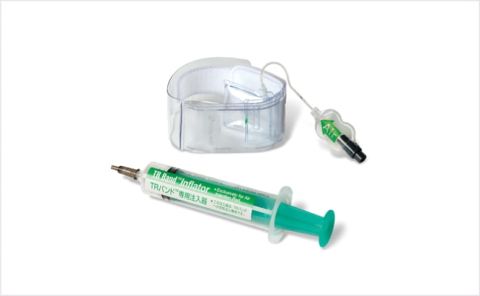
Through the transparent structure designed for visual control and selective compression of the radial artery to allow blood return and preserve patency, TR Band™ assists in maintaining radial artery patency at the time of hemostasis in order to prevent future radial artery occlusion.4,5
How to start R2V™ (Radial to Visceral)
Are concerns and solutions ready?
The problems and solutions that can be considered at each step of Puncture,
Sheath Insertion, Catheter Insertion, and Hemostasis are organized
in an easy-to-understand manner.