The year 2022 marks the 30th anniversary since Dr. F. Kiemeneij performed the first transradial intervention (August 1992).
In celebration of this milestone, Terumo Interventional Systems features the history of the transradial approach, which has evolved together with our customers. We continue to advance a radial first approach as our commitment to improving the quality of life of our patients.
30 Years of Transradial Access:Pushing the boundaries in cardiology & beyond
For 30 years, Terumo Interventional Systems has focused on bringing radial access to the forefront of clinical practice.Our radial access product portfolio, clinical research, and collaborative educational programs have helped thousands of healthcare providers transform coronary care. Looking to the future, we remain fully committed to improving patients‘ quality of life by expanding radial access as the first choice for interventional procedures.
1992
First Trans-radial Intervension
First trans-radial angiography/intervention in cardiology
Dr. Campeau (Canada, 1989)
Dr. Kiemeneij (The Netherlands, 1992)
Dr. Wu (Taiwan, 1993)
Dr. Saito (Japan, 1995)
2000
Product Launch
RADIFOCUS Introducer II M coat
RADIFOCUS Optitorque
TR Band
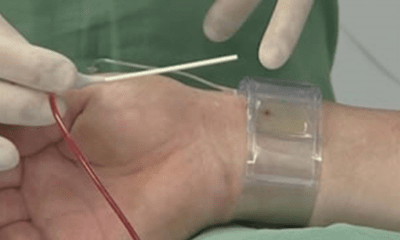
Trans-radial procedures
First trans-radial procedure in various fields
Neuro angioplasty (2000)
Renal stent (2001)
TACE (2002)
Neuro coil (2004)
Iliac stent (2005)
Expansion of Trans-radial procedures in the Europe Union
First PCR Live transmission by Dr. Saito
Massy TRI Course (France)
Publication
1st clinical study on Radifocus™ Introducer II Mcoat™ & the benefits of hydrophilic coating on TRI
Saito S. et al. Catheter Cardiovasc Interv 2002;56:328-32
Usefulness of hydrophilic coating on arterial sheath introducer in transradial coronary intervention - Saito - 2002 - Catheterization and Cardiovascular Interventions - Wiley Online Library
Clinical study on Radifocus™ Introducer II Mcoat™ & its benefits on patient discomfort following TRI
Kiemeneij F. et al. Catheter Cardiovasc Interv 2003;59:161-164
Hydrophilic coating aids radial sheath withdrawal and reduces patient discomfort following transradial coronary intervention: A randomized double‐blind comparison of coated and uncoated sheaths - Kiemeneij - 2003 - Catheterization and Cardiovascular Interventions - Wiley Online Library
2005
Product Launch
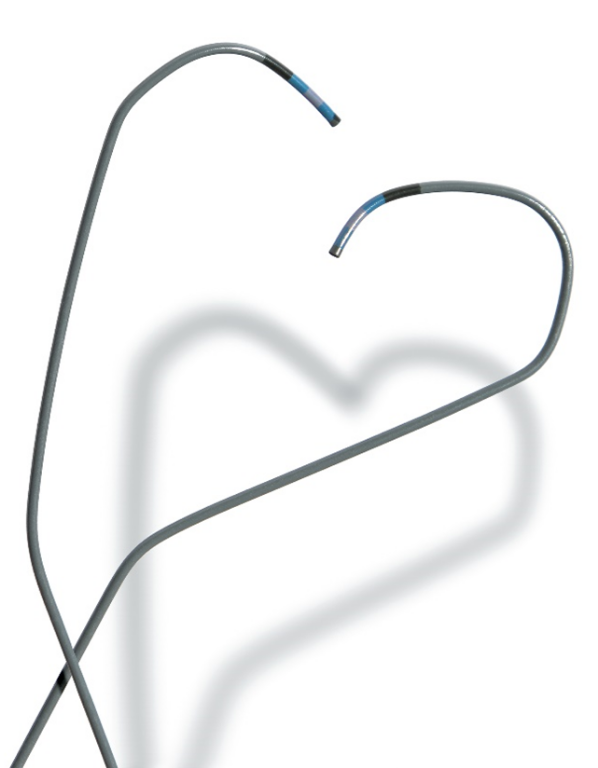
Heartrail II Guiding Catheter Ikari Curve
1.5 mm J-TIP Radifocus™ Guide Wire M / Baby J

1.5 mm J-TIP Radifocus™ Guide Wire M
Terumo Activity
China, Korea, Japan friendship TRI seminar was held

Brazil-Japan 100 years anniversary TRI seminar was held

Training tools and simulators were developed

Trans-radial procedures
Expansion of trans-radial procedures in the United States, Asia, and Latin America
Publication
1st clinical study on Optitorque™'s universal shape TIGER II & its benefits for transradial angiography
Kim S-M et al. Int J Cardiovasc Imaging 2006;22:295-303
Novel diagnostic catheter specifically designed for both coronary arteries via the right transradial approach | SpringerLink
MORTAL Study demonstrates a reduction in mortality and transfusion rate with transradial access (PCI all-comers)
Chase AJ et al. Heart 2008;94:1019–25
Association of the arterial access site at angioplasty with transfusion and mortality: the M.O.R.T.A.L study (Mortality benefit Of Reduced Transfusion after percutaneous coronary intervention via the Arm or Leg) | Heart (bmj.com)
2010
Product Launch
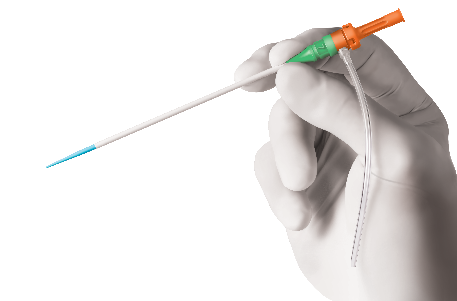
Glidesheath Slender
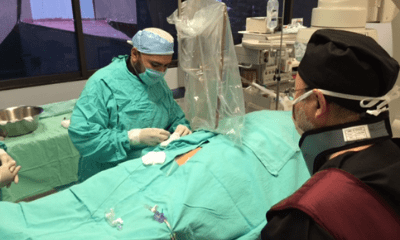
Trans-radial procedures
Trans-radial intervention training for physicians in Latin America conducted through public-private partnership to contribute to improved patient quality of life and reduced healthcare expenditures.
ESC/EACTS 2014 guidelines: Class IIa A recommendation for patients with STEMI ACS -
Kolh P et al. Eur J Cardiothorac Surg 2014;46:517–92
PCI consensus document recommends aiming for the highest possible transradial rate for optimal outcomes
Rao S.V. et al. Catheter Cardiovasc Interv. 2014;83:228-36
Publication
1st clinical study on 6Fr Glidesheath Slender™ & its use for transradial coronary angiography and PCI
Aminian A. et al. Catheter Cardiovasc Interv 2014;84:436-442
2015
Trans-radial procedures
ESC 2015 Guidelines: Class 1 A recommendation for patients with non-STEMI -
Roffi M et al. Eur Heart J 2016;37:267–315.
ESC/EACTS 2018 Guidelines: Class 1 A recommendation for patients with STEMI ACS
Ibanez B et al. Eur Heart J 2018;39:119–7
ESC/EACTS 2018 Guidelines: Class 1 A recommendation
Neumann FJ et al. Eur Heart J 2019;40:87–16
Radial Access for Visceral Intervention (R.A.V.I)
Radial to Peripheral (R2P)
Distal radial approach
ESC 2019 guidelines: Class I B recommendation in elderly patients
Knuuti J et al. Eur Heart J 2020;41:407–77
International Consensus Paper on Best Practices for the pr ion of radial artery occlusion
Bernat I et al. J Am Coll Cardiol Interv 2019;12:2235–46.
Terumo Activity
Partnered with Euro PCR and conducted training.
Publication
The MATRIX Access trial demonstrates clinically relevant reduction in all-cause mortality with transradial access in patients with STEMI or non-STEMI ACS
Valgimigli M et al. Lancet 2015;385:2465–76
1st clinical study on 5Fr Glidesheath Slender™ & its use for transradial coronary angiography and PCI
Yoshimachi F. et al. Cardiovasc Interv and Ther 2016;31:38-41
RAP and BEAT trial compares 6Fr Glidesheath Slender™ with a standard 5 Fr sheath for transradial coronary angiography and PCI
Aminian A. et al. EuroIntervention. 2017;13:e549-e556.
CRASOC I, II, and III studies featuring with TR Band™ demonstrates the benefits of a gentle and short hemostasis for transradial coronary angiography and PCI
Dangoisse V. et al Am J Cardiol. 2017;120:374-379
1st clinical study on 7Fr Glidesheath Slender™ & its use for complex transradial PCI
Aminian A. et al. Catheter Cardiovasc Interv 2017;89:1014-1020
RAP and BEAT trial substudy featuring with TR Band™ demonstrates the benefits of short hemostasis time on radial artery patency
Aminian A. et al Catheter Cardiovasc Interv. 2018 ;92:844-851
Clinical trial on Optitorque™'s universal shape TIGER II & its benefits over conventional two-catheter strategy
Xanthopoulou I et al. EuroIntervention 2018;13:1950-1958
Clinical study featuring Optitorque™'s universal shape TIGER I demonstrates a positive impact on coronary catheterization performance and economic costs
Costa-Mateu J et al. Arq Bras Cardiol 2019;113:960-968
Clinical study featuring 7 Fr Glidesheath Slender™ supports its uses for left distal transradial approach & CTO intervention
Gasparini G.L. et al. EuroIntervention. 2019;15:126-128
2020
Product Launch
RADIFOCUS Glidecath R.A.V.I. MG1/MG2 Shape
Trans-radial procedures
ACC/AHA/SCAI Decision to Designate Radial Access Class 1, Level of Evidence A
2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines | Journal of the American College of Cardiology (jacc.org)
Publication
COLOR Trial featuring 7Fr Glidesheath Slender™ demonstrates that TRA is superior to TFA in complex large bore PCI
Meijers T.A. et al. JACC Cardiovasc Interv.2021;14:1293-1303
1st clinical study on Heartrail II's Ikari left curve & its benefits door-to-balloon time for PCI STEMI patients
Lee K.H. et al. Catheter Cardiovasc Interv. 2021 May 31. Online ahead of print
DISCO RADIAL trial featuring Glidesheath Slender™ designed to investigate the benefits of dTRA over cTRA on RAO rates at discharge
Terumo Press Release 21 January 2020. Available at: https://www.terumo.com/pressrelease/detail/20200121/514/; Glidesheath Slender™ product
information https://www.terumois.com/products/access/glidesheath-slender.html; all accessed May 2020

TRI 30th Anniversary Newsletter
Radial approach topics are regularly available and include the history, clinical outcomes, benefits, dedicated devices, guidelines, and other topics.